1. INTRODUCTION
Medicinal plants encompass a wide range of species that have been used by diverse cultures over thousands of years for treatments and disease prevention. These plants are rich in chemically diverse compounds, including alkaloids, flavonoids, terpenes, and phenolics, which contribute to a wide spectrum of therapeutic properties such as antibacterial, antiviral, antifungal, anti-inflammatory, analgesic, immune-boosting, and calming effects (Parham et al., 2020). The history of medicinal plant use dates back to times before written records, and ancient civilizations heavily relied on the curative power of plants. Traditional systems such as Ayurveda, Siddha, and other indigenous healing practices have long utilized these botanicals for therapeutic purposes (Miranda, 2021).
Medicinal plants grow in a variety of ecosystems, from dense forests and high mountains to arid deserts and humid wetlands (Chen et al., 2016). Many of these plants support specific physiological functions, aiding in the health of the liver and digestive, respiratory, and cardiovascular systems (Parham et al., 2020). In recent years, there has been a resurgence of interest in natural and holistic approaches to health, leading to renewed appreciation for medicinal plants and their potential health benefits.
Scientific advancements have enabled researchers to delve deeper into the composition and bioactivity of these plants, which helped to substantiate their traditional uses (Welz et al., 2018). Bioactive compounds are naturally occurring substances with physiological effects that have garnered attention for their therapeutic potential, including antioxidant, anti-inflammatory, and anti-cancer properties (Samtiya et al., 2021; Yuan et al., 2022). These compounds are primarily found in the secondary metabolites of plants, which have demonstrated notable health benefits in both humans and animals. Research continues to support the integration of medicinal plants into modern healthcare systems as complementary and alternative medicines (Fokunang et al., 2011).
In addition to terrestrial plants, aquatic resources, both flora and fauna from marine and freshwater environments, have emerged as promising sources of bioactive compounds. These compounds have demonstrated a broad range of biological activities, making them important subjects of investigation in the biomedical sciences (Karthikeyan et al., 2022). Studies show that aquatic plant-derived bioactives possess antimicrobial, antioxidant, antithrombotic, antihypertensive, and anti-inflammatory activities. These properties are essential in the development of new multifaceted drugs and treatments for infectious diseases (Khan et al., 2019; Salehi et al., 2020; Vaou et al., 2021).
The growing interest in bioactive compounds from aquatic organisms has led to the advancement of bioprospecting a systematic exploration of biological resources to discover compounds with potential medical applications. In aquatic bioprospecting, researchers search for unique chemical structures that can be developed into innovative drugs, therapies, and health products (Pushpangadan et al., 2018).
One aquatic plant of particular interest is Echinodorus cordifolius, commonly known as the spade-leaf sword or creeping burhead. It belongs to the Alismataceae family and thrives in wetland environments (Fassett, 1955). This semi-aquatic plant has been widely recognized for its phytoremediation abilities and its capacity to absorb and detoxify pollutants such as heavy metals and organic compounds from soil and water. However, beyond its ecological utility, E. cordifolius remains relatively underexplored for its biomedical properties.
There is limited research investigating the antimicrobial and antioxidant properties of E. cordifolius, despite its promising bioactive potential. As such, examining the chemical makeup of this plant could uncover important compounds with therapeutic significance. The present study evaluated the biological activity of E. cordifolius extracts, with focus on antimicrobial and antioxidant effects. Through methodological extraction and chromatographic separation, the study isolated different fractions of E. cordifolius and then analyzed using High-Resolution Mass Spectrometry (HRMS), revealing several key compounds. These findings suggest that E. cordifolius is not only a phytoremediator but also a potential source of bioactive agents that could contribute to drug development and therapeutic innovations.
2. MATERIALS AND METHODS
2.1. Plant sample collection
Healthy leaves of E. cordifolius were collected and identified from the smart medicinal garden of Kristu Jayanti College campus, Bangalore. The collected leaves were thoroughly washed under running tap water and by air dried. They were then placed in a hot air oven and dried at 60 °C for 24 h. The dried leaves were then powdered in a mixer grinder and weighed using analytical balance. Around 30.83 g of the sample powder was obtained for further analysis.
2.2. Extraction of bioactive compounds from leaves of Echinodorus cordifolius
The Soxhlet extraction was performed according to the standard method by Azmir et al. (2013). For this process, 14 g of the E. cordifolius leaf powder was packed in Whatman paper and soaked overnight in 500 mL of methanol. After 1 day of the soaking process, the Soxhlet apparatus was run for 15 cycles. After extraction was complete, the crude extract was obtained by evaporating in a water bath at 70 °C. Following drying, the crude extract was acquired and subsequently stored at 4 °C until further analysis.
2.3. Phytochemical screening of Echinodorus cordifolius extracts
The phytochemical analysis was conducted following standard procedures outlined by Gul et al. (2017). The specific tests performed are detailed below:
2.3.1. Test for carbohydrates (Benedict’s Test)
A color change from clear blue to brick red, accompanied by precipitation, is observed when 0.5 mL of the sample is mixed with Benedict’s reagent, confirming the existence of carbohydrates.
2.3.2. Test for proteins (Ninhydrin Test)
The appearance of a violet color when 0.5 mL of the sample is mixed with ninhydrin reagent confirms the existence of proteins.
2.3.3. Test for phenols
The appearance of a blue–green or black color when 2 mL of ferric chloride is mixed with 0.5 mL of sample confirms the existence of phenols.
2.3.4. Test for terpenoids
The formation of a reddish brown color when 0.5 mL of the sample is mixed with chloroform and concentrated sulfuric acid confirms the existence of terpenoids.
2.3.5. Test for alkaloids (Hager’s Test)
The formation of yellow-colored precipitates when the sample is treated with Hager’s reagent (saturated picric acid solution) confirms the existence of alkaloids.
2.3.6. Test for flavonoids (Alkaline Reagent Test)
Sample extracts mixed with 0.5 mL of 2.0% NaOH mixture results in the formation of a concentrated yellow color, which turned colorless upon addition of diluted acid, confirms the existence of flavonoids.
2.4. Separation of bioactive compounds of Echinodorus cordifolius extract by column chromatography
The bioactive components in the crude extract of E. cordifolius were separated and purified using column chromatography, as explained by Bajpai et al. (2016). A long cylindrical glass column was packed firmly with 60 mm mesh silica gel using hexane as nonpolar solvent to serve as the stationary phase. One gram of the plant extract was carefully added to the top of the column after being dissolved in hexane. For successful separation, a gradient solvent system was employed, beginning with hexane and working up to more polar solvents. Depending on their affinity for the stationary and mobile phases, the compounds traveled through the column at varying rates as the solvents went through it under gravity. Individual components in the mixture were separated as a result of this differential mobility. As the solvent eluted through the column, fractions were methodically gathered and labeled for additional examination. To ensure maximum elution of chemicals with different polarities from the plant extract, the solvent selection and order were crucial. The ratios of polar to nonpolar solvents employed in the elution procedure are shown in Table 1. The amount of sample being purified was taken into consideration when adjusting the volume of each solvent. The effective separation of bioactive substances for further characterization and biological activity evaluation was made possible by this approach.
Table 1
Gradient solvent ratios employed in column chromatography.
2.5. Antioxidant activity of the crude extract of Echinodorus cordifolius
The antioxidant activity of E. cordifolius was assessed using the DPPH free radical scavenging assay (Nićiforović et al., 2010). For this purpose, 2,2-diphenyl-1-picryl-hydrezyl (DPPH) was utilized as the free radical. A 0.1 M concentration of DPPH was prepared by dissolving 4 mg in 100 mL of methanol in a volumetric flask, which was then covered with foil and stored in a cool, dark place. Different concentrations of the sample were prepared by dissolving 1 g of the crude extract in 25 mL of methanol and incubating for 30 min at 37 °C in a water bath. Following this, the mixture was centrifuged at 6000 rpm for 15 min and filtered using Whatman paper. Subsequently, various concentrations of the extract were prepared (4, 8, 12, 16, and 20 mg/mL) by diluting with methanol. All readings were taken in triplicate, and the average was calculated. Ascorbic acid served as the standard antioxidant. Absorbance at 517 nm was measured with a UV spectrophotometer. Assay results were statistically analyzed, and data were reported as mean ± standard deviation of triplicate trials.
2.6. Antimicrobial activity screening of Echinodorus cordifolius extract
The antimicrobial activity of the 13 eluted samples obtained from column chromatography was determined using the well diffusion assay according to Balouiri et al. (2016). The assay targeted gram-positive microorganisms, specifically Staphylococcus aureus and Bacillus subtilis, as well as gram-negative microorganisms such as Escherichia coli and Pseudomonas fluorescens, sourced from MTCC. Cultures of these microorganisms were propagated using Mueller–Hinton agar. To conduct the assay, wells were created on agar plates using a sterile pipette. The eluted samples were dissolved in 1 mL of 0.1% dimethyl sulfoxide (DMSO), and 100 µL of each sample was added to the respective wells. Tetracycline served as the positive control, while 0.1% DMSO was used as the negative control. Subsequently, the plates were incubated at 37 °C for 24 h to allow for bacterial growth and potential inhibition.
2.7 High-Resolution Mass Spectrometry
High-Resolution Mass Spectrometry (HRMS) was used to assess the bioactive ingredients found in the eluted plant extract samples. The samples were diluted with LC-MS grade methanol and filtered through a 0.22 µm syringe filter before analysis. A Thermo Scientific Q ExactiveTM Orbitrap mass spectrometer with an electrospray ionization (ESI) source was used for HRMS analysis using Thermo Dionex Ultimate 3000 UHPLC system. At 30 °C, a C18 reverse-phase column was used to produce chromatographic separation. Both positive and negative ion modes were used by the device. Complete MS scans were captured between m/z 100 and 1000. Data processing and analysis were carried out using Xcalibur software, and tentative identification of compounds was based on mass measurements, isotopic distribution, and comparison with literature and compound databases such as METLIN, MassBank.
3. RESULTS AND DISCUSSION
3.1. Extraction and separation of bioactive compounds from Echinodorus cordifolius
3.1.1. Extraction of the bioactive compounds using Soxhlet technique
The extraction of bioactive compounds from the leaves of E. cordifolius (Figure 1A) was carried out using a Soxhlet apparatus, with methanol serving as the solvent (Figure 1B). The yields of the extracts and the resultant biological activities of the plant materials were significantly influenced by the choice of extracting solvent. The antibacterial and antioxidant compounds in the plant extract vary greatly in their polarity, solubility, and chemical properties within a given solvent. Polar solvents are commonly employed for the recovery of polyphenols and alkaloids from plant matrices (Sultana et al., 2009). Moreover, methanol exhibits relatively high solubility for a wide spectrum of compounds, being capable of dissolving both polar and nonpolar substances. Consequently, methanol is well-suited for extracting a diverse array of bioactive compounds with varying polarities (Altemimi et al., 2017). Studies have demonstrated that methanolic extracts from various plants, such as Severini abuxifolia (Truong et al., 2019), Limnophila aromatica (Do et al., 2014), Euphorbia aegyptiaca, Cissus quadrangularis, Francoeuria crispa, and Grewia tenax (Adam et al., 2019), have resulted in the highest extraction yields and content of phenolic compounds, flavonoids, alkaloids, and terpenoids. The extraction process adhered to the fundamental principles of all solvent extraction processes, encompassing several key steps: solvent penetration into the solid matrix, dissolution of the target compounds in the solvent, diffusion of the compounds out of the solid matrix, and collection of the extracted compounds (Azmir et al., 2013; Smith, 2003). After concentration, about 5 g of crude extract was recovered.
3.2. Phytochemical screening of Echinodorus cordifolius
The results of the phytochemical screening of E. cordifolius is as depicted in Table 2 and Figure 2. It was observed that carbohydrates, proteins, phenols, terpenoids, alkaloids, flavonoids, saponins, and steroids were present in the leaf extract of the plant and tested negative for the iodine test. There have been no previous studies on the phytochemical compounds of E. cordifolius, however these findings are consistent with the results of species belonging to the same genus as found by Brugiolo et al. (2010), which showed the presence of alkaloids, flavonoids, saponins, and tannins, in addition to terpenes, triterpenes, and cardiotonic glycosides. Similar studies by Bonetti et al. (2020) and Gasparotto et al. (2019) in phytochemical analysis utilized hydroalcoholic extract from the leaves of E. grandiflorus and identified various classes of secondary metabolites, including tannins, flavones, saponins, and alkaloids. These findings support the results of this research.
3.3. Antioxidant activity of crude extracts
The IC50 value is a critical parameter in evaluating the antioxidant capacity of substances, particularly in DPPH radical scavenging assays. IC50 represents the concentration of a substance required to inhibit 50% of the initial DPPH radicals, with lower IC50 values indicating stronger antioxidant activity. For the crude methanolic extract of E. cordifolius, the IC50 value was found to be 4.66 mg/mL, which is lower than the IC50 value of 5.71 mg/mL for standard ascorbic acid. This indicates that the E. cordifolius extract exhibits superior free radical scavenging activity compared to ascorbic acid, suggesting higher antioxidant potency. The effectiveness of the crude methanolic extract can be attributed to the presence of various bioactive compounds, particularly polyphenols and flavonoids, which are known for their antioxidant properties (Altemimi et al., 2017). These findings align with those of Gasparotto et al. (2019), Lunardi et al. (2014) and Berwig (2014), who reported IC50 values for E. grandifolius ranging from 0.61 to 5.5 mg/mL, demonstrating significant antioxidant potential. Furthermore, the study by Mahdi-Pour et al. (2012) supports the notion that E. cordifolius exhibits potential antioxidant activity due to its rich composition of secondary metabolites, such as polyphenolic compounds and alkaloids, detected during phytochemical screening. Overall, these results suggest that the methanolic extract of E. cordifolius has a promising potential for use as a natural antioxidant, possibly even outperforming standard antioxidants like ascorbic acid.
3.4. Separation of bioactive compounds using column chromatography
To achieve further separation and purification of the crude extract, column chromatography (Figure 2) was employed, utilizing a set of 13 different solvent ratios that resulted in 13 distinct elutions. This strategy was observed in the research carried out by Bajpai et al. (2016), Ngo and Chua (2019), and Gini and Jothi (2018), which employed a similar technique using a column packed with silica gel and a gradient solvent system ranging from nonpolar to highly polar solvents. Following the chromatographic process, the 13 eluted samples were systematically collected and assigned labels as E. cordifolius A (EC-A), EC-B, EC-C, and so on. Subsequently, the eluted samples were subjected to concentration using a water bath, resulting in a reduction of solvent volume and an increase in compound concentration. These concentrated fractions were carefully stored, preserving their integrity and ensuring their availability for further analysis and characterization. This method enabled the separation and isolation of specific compounds present in the crude extract of E. cordifolius, providing a collection of individual fractions that could be studied independently. The subsequent analysis and characterization of these fractions would facilitate the identification of bioactive compounds and enable a more comprehensive understanding of their chemical properties and potential applications.
3.5. Antimicrobial activity of the extracts
Out of the 13 eluted fractions obtained using varying solvent ratios through column chromatography, fractions EC-B and EC-C demonstrated notable antimicrobial activity against E. coli, exhibiting zone of inhibition measuring 8.5 and 6.5 mm, respectively (Figure 3A). Fractions EC-G and EC-I exhibited activity against P. fluorescens with inhibition zones of 8.4 and 10.3 mm, respectively (Figure 3B). Remarkably, fraction EC-I also showed strong inhibition against B. subtilis and S. aureus, measuring 13.2 (Figure 3C) and 12.9 mm, respectively (Figure 3D). Among all tested fractions, EC-I displayed the broadest and most potent antimicrobial spectrum, effectively targeting gram-positive and gram-negative bacteria as summarized in Table 3.
Figure 3
Antimicrobial activity of purified extracts against (A) Escherichia coli, (B) Pseudomonas fluorescens, (C) Bacillus subtilis, and (D) Staphylococcus aureus.
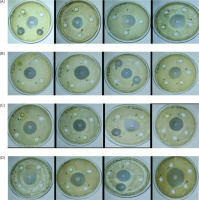
Table 3
Antimicrobial activity of Echinodorus cordifolius.
The EC-I fraction, eluted using a chloroform: ethanol (5:1) solvent system, appears to be enriched with potent bioactive compounds such as quercetin, chlorogenic acid, galloyl-HHDP-glucose, and pentacyclic triterpenol methyl esters, all of which are known for their antimicrobial properties. These findings agree with previous reports on the antimicrobial potential of related species. For instance, the evaluation of the aqueous and methanolic leaf extracts of Erica grandiflora (a species from the same genus) by Bonetti et al. (2020) reported significant antibacterial activity against pathogenic S. aureus using the disc diffusion method. Similar antimicrobial effects of flavonoid- and polyphenol-rich plant extracts have been widely documented, supporting the role of such phytochemicals in disrupting the bacterial cell walls and inhibiting essential metabolic pathways (Cushnie & Lamb, 2005; Daglia, 2012).
3.5. Identification and characterisation of the bioactive compounds from Echinodorus cordifolius
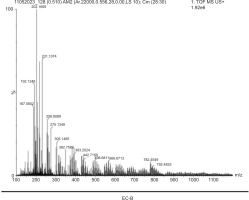
High-Resolution Mass Spectrometry analysis was chosen as a rapid analytical tool to obtain the bioactive compound profiles of the eluted samples that exhibited antimicrobial activity, namely EC-B, EC-C, EC-G, and EC-I. The analysis was performed in both positive and negative ionization modes. The substances and outcomes were identified and assessed based on the obtained mass spectra. The m/z values provided represent the approximate mass-to-charge ratios of the respective compounds. The nature of each compound accurately describes its properties and activities. Around 77 diverse chemical constituents have been identified in all the eluted fractions, which belong to various classes, including flavonoids, alkaloids, terpenes, quinones, carboxylic acids, phenolic acids, and other components. The bioactive compounds in EC-B comprise both alkaloids and flavonoids and were identified as palmatine, methyl gallate, afzelechin/epiafzelechin, quercetin, and 7-angeloyl at m/z 202.16, 187.08, 275.13, 305.14, and 382.75, respectively (Table 4; Figure 4), in both positive and negative modes. These flavonoids, such as quercetin, are considered essential components in nutraceutical, pharmacological, and medicinal applications and are featured to have antioxidant, anti-inflammatory, and antitumor properties (Dahibhate et al., 2022; Panche et al., 2016).
Table 4
Bioactive compounds profile of EC-B.
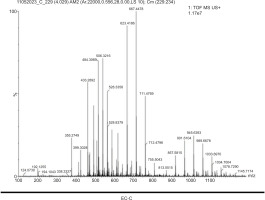
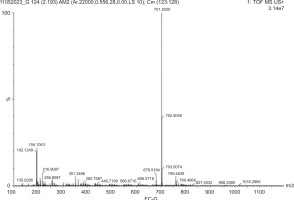
Furthermore, the bioactive profile of EC-C as identified by mass spectrophotometry revealed prominent peaks at m/z 355.27, 433.28, 484.30, and 528.83. These peaks correspond to known phytochemicals such as chlorogenic acid, vitexin, galloyl-HHDP-glucose, and kaempferol 3 (p-coumaroylglucosyl), respectively. These compounds are well documented for their diverse range of biological activities, including antioxidant, antimicrobial, antibiofilm, and anti-inflammatory effects (Table 5; Figure 5). The bioactive compound profile of EC-G exhibited distinct peaks at m/z 351.24, 382.75, 440.71, and 679.51, corresponding to chlorogenic acid, 7-angeloyl, dimeric xanthone, and pentacyclic triterpenol methyl ethers, respectively. These phytochemical components possess antiviral, antibacterial, antifungal, antitumor, antiallergic, and antioxidant properties (Table 6; Figure 6).
Table 5
Bioactive compounds profile of EC-C.
Table 6
Bioactive compounds profile of EC-G.
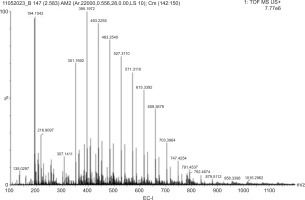
Among the tested extracts, EC-I exhibited the highest antimicrobial potency attributed to the rich composition of polyphenols, flavonoids, and tannins. Mass spectrometric analysis revealed the prominent peaks at m/z 307.14, 351.16, 439.22, 483.25, 527.28, and 615.33 corresponding to quercetin, chlorogenic acid, pentacyclic triterpenol methyl ethers, galloyl-HHDP-glucose, kaempferol 3, and quercetin-O-galloyl-glucoside (Table 7; Figure 7), respectively. These compounds are known for their potent antimicrobial, antioxidant, and anti-inflammatory activities.
Table 7
Bioactive compounds profile of EC-I.
The identified bioactive compounds in each fraction exhibited antimicrobial and antioxidant activities in our study. According to the literature, these compounds are have a wide range of biological activities including antioxidant, anticancer, antiapoptotic, antiallergic, antibacterial, antifungal, antiviral, and antiinflammatory effects (Bakr et al., 2017; Das et al., 2022; Doshi et al., 2015; Enomoto et al., 2019; Kachkoul et al., 2020; Lou-Bonafonte et al., 2012; Marulasiddaswamy et al., 2021; Mugaranja & Kulal, 2022; Oyo-Ita et al., 2010; Sapkota et al., 2022; Shahrivari et al., 2024; Shawky et al., 2021; Souza et al., 2023; Wani et al., 2023). Further research and analysis are required to determine the specific mechanisms and potential synergistic effects of these compounds concerning their observed activities.
4. CONCLUSION
Drug research and discovery are greatly aided by the wide range of chemically diverse bioactive compounds present in medicinal plants. Natural plant chemicals, which exhibit diverse modes of action from synthetic medications, are the source of many contemporary pharmaceuticals. These natural remedies may have fewer adverse effects, decreased toxicity, and increased efficacy. One of the most important methods for detecting the possible lead molecules for upcoming medication development is screening medicinal plants for bioactive substances with antibacterial and antioxidant qualities.
A variety of secondary metabolites were successfully recovered using methanol, which is well-known for its capacity to dissolve both polar and nonpolar substances. Proteins, carbohydrates, phenols, terpenoids, alkaloids, flavonoids, saponins, and steroids were detected by phytochemical screening of the crude methanolic extract. These results are consistent with earlier research on related species. The crude extract’s antioxidant activity showed a lower IC50 value than that of ascorbic acid, indicating a robust ability to scavenge free radicals, most likely because of polyphenols and alkaloids. Among the different fractions obtained by column chromatography, EC-I exhibited the strongest antibacterial activity against S. aureus, B. subtilis, and P. fluorescens. The antibacterial, antioxidant, and anti-inflammatory properties of flavonoids, polyphenols, tannins, and alkaloids were among the important substances found in these fractions by HRMS. This work underscores the need to examine underused plant species for their potential involvement in contemporary medicine and the importance of medicinal sources of bioactive compounds in E. cordifolius bioprospecting in aquatic habitats. Further studies will be required to isolate these compounds in pure form, determine their mechanisms of action, and explore their full therapeutic potential in clinical contexts. Eventually, these extracts might result in the creation of functional foods, nutraceuticals, or medications.
ETHICAL APPROVAL
The author declares that this study does not involve any human participants, their data, biological material, or animals.