1. INTRODUCTION
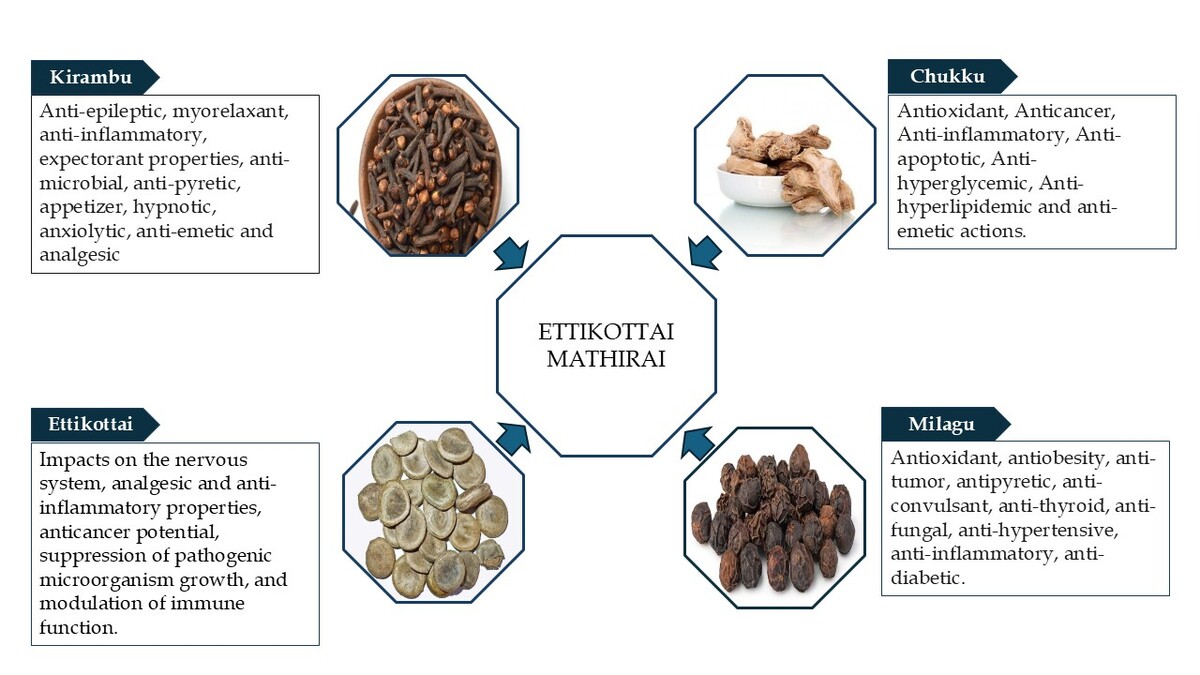
Siddha medicine, a traditional Indian healing system, is a time-honored and indigenous healing system primarily practiced in South India. It emphasizes on preventive care, health promotion, and rejuvenation to sustain the overall well-being. This system is based on the tridosha theory, which maintains that health depends on the balance of three fundamental humors: vatham, pitham, and kabam. Any disruption in their equilibrium is believed to result in illness (Roshan et al., 2023). The Siddha medicine is split into two groups, internal medicine and external medicine. One of the internal medications, that is, mathirai (pills), has 1-year shelf life. Leaves and fruit juices of Kabasura Kudineer are used to triturate raw medications. They are dried, stored, and sized differently (Sambasivampillai, 1931). Herbs, metals, minerals, and animal products are widely employed in the Siddha system of Indian medicine to make medications that treat a variety of illnesses. According to Nam Nattu Vaithiyam, Ettikottai Mathirai (EM) is a polyherbal formulation indicated for 18 types of soolai (pain), including lumbar pain. EM comprises five herbal drugs: milagu (Piper nigrum), chukku (Zingiber officinale), ettikottai (Strychnos nux-vomica), kirampu (Syzygium aromaticum), and elumichai (Citrus limon).
The primary component of EM is Strychnos nux-vomica, the evergreen strychnine tree native to India and Southeast Asia, belonging to the Loganiaceae family. In traditional Oriental medicine, detoxification techniques were employed to minimize the toxicity of nux-vomica. The seeds undergo specific processing methods to reduce or neutralize the toxic effects of their alkaloid content. Through purification procedures (suthimurai), including soaking and heating, the toxic compounds may undergo chemical transformations while preserving the therapeutic benefits of seeds (Wu et al., 2007). Strychnine, a predominant alkaloid in nux-vomica (constituting 40–50%), is recognized as a highly toxic component. In humans, the lethal dosage is estimated to range between 30 mg and 120 mg (Makarovsky et al., 2008). If administered in minimal amount, it functions as a stimulant, laxative, and remedy for digestive disorders whereas excessive intake leads to toxicity. Pharmacological studies indicate that Strychnos nux-vomica exhibits anticancer, antimicrobial, anti-inflammatory, and antioxidant properties.
This review describes phytochemicals, pharmacological action, scientific review, and medicinal uses of each ingredient constituting EM.
2. MATERIAL AND METHODS
2.2. Preparation of Ettikottai Mathirai
All the ingredients were roasted and crunched into a fine powder. The powder was further pestled by adding lemon juice until to make pills (Pillai, 2012).
Dosage: 65–130 mg, twice a day (b.i.d.)
Adjuvant: warm water or buttermilk
The components of EM are detailed in Table 1.
Table 1
Ingredients of EM.
2.3. Purification of Raw Drugs
Chukku: Soaked in limestone water for 3 hours and dried. The outer layer is peeled off (Samuel, 2014).
Milagu: Soaked in sour buttermilk for 1–1¼ hours and then fried (Samuel, 2014).
Kirampu: Buds of the flowers were removed and fried (Samuel, 2014)
Ettikottai: Remove the outer skin and cotyledons of ettikottai and deep-fried in ghee (Samuel, 2014).
2.4. Review of Ettikottai Mathirai
The chemical composition and associated pharmacological properties of EM’s ingredients are outlined in Table 2.
Table 2
Chemical composition and pharmacological properties of ingredients of EM.
| S. No. | Plant name | Action | Chemical constitution | Pharmacological activity |
|---|---|---|---|---|
| 1. | Chukku | Stimulant, carminative, and stomachic (Mudaliyar, 2013) | Monoterpenoids (B-phellandrene, camphene, cineole, geraniol, curcumene, citral, terpineol, borneol, cineole, geranyl acetate, limonene, linalool), sesquiterpenoids, and zingiberol (Singh & Singh, 2019) | Antioxidant, Anticancer, Anti-inflammatory, Anti-apoptotic, Anti-hyperglycemic, Anti-hyperlipidemic, and anti-emetic actions. (Rehman et al., 2011; Shahrajabian 2019) |
| 2. | Milagu | Stimulant, carminative, acrid, antiperiodic, rubefacient, resolvent, anti-vatha, and antidote (Mudaliyar, 2013) | Piperonal (2E,4E)-N-isobutyl-2,4-decadienamide. Piperine, piperanine, piperettine, piperylin A, piperolein B, pipericine, flavonoids, alkaloids, and phenolic amides (Damanhouri & Ahmad, 2014) | Antioxidant, anti-obesity, antitumor, antipyretic, anticonvulsant, anti-thyroid, antifungal, antibacterial, insecticidal, hepatoprotective, anti-asthmatic, larvicidal, antihypertensive, anti-inflammatory, anti-diabetic, antidiarrheal, bioavailability enhancer, immunomodulatory, antiepileptic, anti-fertility, GI-stimulant, lipid metabolism accelerator, anticancer, CNS stimulant, diuretic, aphrodisiac, blood purifier, and anti-platelet (Ashok Kumar et al., 2021; Saleem et al., 2022) |
| 3. | Kirampu | Antispasmodic, carminative, and stomachic (Mudaliyar, 2013) | 3-Allyl-6-methoxyphenol; eugenol acetate; 2-pentanone, 4-hydroxy-4-methyl, and caryophyllene (Uddin et al., 2017) | Antimicrobial, antipyretic, appetizer, hypnotic, anxiolytic, antiemetic, analgesic, decongestant, antimicrobial, antiepileptic, myorelaxant, anti-inflammatory, and expectorant properties (Srivastava & Singh, 2023) |
| 4. | Ettikottai | Antiseptic, carminative, purgative, tonic, stimulant, diuretic (Mudaliyar, 2013) | Strychnine, brucine, pseudo-strychnine, pseudo-brucine, secoxyloganin, caffeic acid, p-hydroxybenzoic acid, p-hydroxyphenyl acetic acid, uvaol, stigmasta-7,22,25-triene-3-ol, lupeol, 11-oxo-α-amyrin palmitate, catechol, maltol, adenosine (Zhang & Tu, 2012) | Impacts on the nervous system, analgesic and anti-inflammatory properties, anticancer potential, suppression of growth of pathogenic microorganisms growth, and modulation of immune function (Rixin Guo et al., 2018). |
| 5. | Elumichai | Refrigerant (Mudaliyar, 2013) | Limonene (84.73%), α-pinene (1.06%), α-terpineol (2.80%), β-myrcene (2.16%), β-pinene (3.36%), terpinene-4-ol (1.16%), and α-terpinolene (2.33%) (L-Jabri & Hossain, 2018) | Anti-inflammatory, antimicrobial, anticancer, and antiparasitic activities (Klimek-Szczykutowicz et al., 2020) |
Scientific properties and review of EM ingredients are summarized in Table 3.
Table 3
Scientific review of the ingredients of EM.
| S. No. | Ingredients | Scientific review |
|---|---|---|
| 1. | Chukku | The analgesic and anti-inflammatory properties of (6)-gingerol, the bioactive pungent compound in ginger, are studied. When administered intraperitoneally at doses ranging from 25 mg/kg to 50 mg/kg, (6)-gingerol significantly reduced the writhing response induced by acetic acid and decreased duration of licking in the late phase of formalin-induced pain. Additionally, at higher doses (50–100 mg/kg), it effectively inhibited paw edema caused by carrageenan. These findings indicate that (6)-gingerol exhibits both analgesic and anti-inflammatory effects (Luo et al., 2005). |
| 2. | Milagu | The analgesic activity of pure piperine, along with hexane and ethanol extracts, was assessed using the tail immersion method. The highest analgesic activity was observed at a dose of 10 mg/kg, compared to the control and standard drug, with the effect intensifying over time and reaching its peak after 60 minutes.Anti-inflammatory compounds primarily work by inhibiting the cyclooxygenase (COX) enzyme, which plays a vital role in prostaglandin synthesis. The study revealed that piperine, at all tested doses, progressively reduced volume of edema over time. Meanwhile, the anti-inflammatory effects of hexane and ethanol extracts of Piper nigrum L. continued to increase up to 120 minutes. These findings suggest that piperine exhibits anti-inflammatory properties by inhibiting prostaglandin release (Tasleem et al., 2014). |
| 3. | Kirambu | The anti-inflammatory properties of eugenol are linked to its ability to inhibit neutrophil and macrophage chemotaxis, suppress prostaglandin synthesis, and reduce the expression of cyclooxygenase-II enzyme (Nejad et al., 2017). Additionally, eugenol dimers have demonstrated chemopreventive potential by downregulating cytokine expression in macrophages (Leem et al., 2011). Owing to its beneficial effects on arthritis, eugenol is proposed as a potential therapeutic agent for managing arthritis (Grespan et al., 2012).Eugenol is found to inhibit action potential conduction in the sciatic nerves (Kozam, 1977). It also suppresses N-methyl-D-aspartate (NMDA) receptors, which play a key role in pain perception (Aoshima & Hamamoto, 1999). Additionally, studies have shown that the oral administration of eugenol produces analgesic effects, which can be reversed by naloxone (Paula-Freire et al., 2013). |
| 4. | Ettikottai | The evaluation of analgesic activity in the total alkaloid fraction revealed that a modified version with a higher brucine content exhibited enhanced pain-relieving effects across various nociception models, including chemical, physical, and thermal stimuli. This suggests the involvement of both central and peripheral mechanisms in pain modulation. Additionally, brucine in the modified total alkaloid fraction demonstrated complete absorption through transdermal administration, leading to improved pharmacological efficacy (Chen et al., 2012).Further studies on brucine and brucine-oxide highlighted their analgesic effects against thermal and chemical stimuli, as observed in hot-plate and writhing tests. Moreover, an analysis of 6-keto-PGF1α levels in the blood plasma of mice with arthritis induced by Freund’s complete adjuvant provided evidence that both central and peripheral pathways contribute to pain regulation (Yin et al., 2003). |
| 5. | Elumichai | The aqueous, alcoholic, and methanolic extracts of Citrus limon have demonstrated significant anti-inflammatory effects in mice with carrageenan-induced and formalin-induced paw edema. A noticeable reduction in volume of paw edema was observed, compared to both control and standard treatments. Additionally, these extracts exhibited analgesic properties in hotplate and tail-flick methods, as evidenced by an increase in mean latency to thermal pain. The presence of certain bioactive compounds in Citrus limon is believed to contribute to pain relief by modulating or inhibiting pathways involving histamine and kinin (Dara et al., 2017). |
Ettikottai Mathirai
3. CONCLUSIONS
The review highlights the growing body of evidence supporting the therapeutic potential of EM. The reviewed studies demonstrate its efficacy in addressing various health conditions, particularly its pharmacological activities, such as anti-inflammatory, analgesic, and antioxidant effects. Specifically, EM has shown promise in the management of pain-related conditions such as soolai (pain), lumbar, and cervical pain. While the available data are promising, it is evident that further clinical research and trials are necessary to confirm the long-term safety and effectiveness of the formulation. Overall, EM holds significant promise as a viable therapeutic option for pain management, warranting continued exploration and scientific validation.
ACKNOWLEDGMENT
The authors acknowledge the support and facilities provided by the National Institute of Siddha, Tambaram Sanatorium, Chennai.