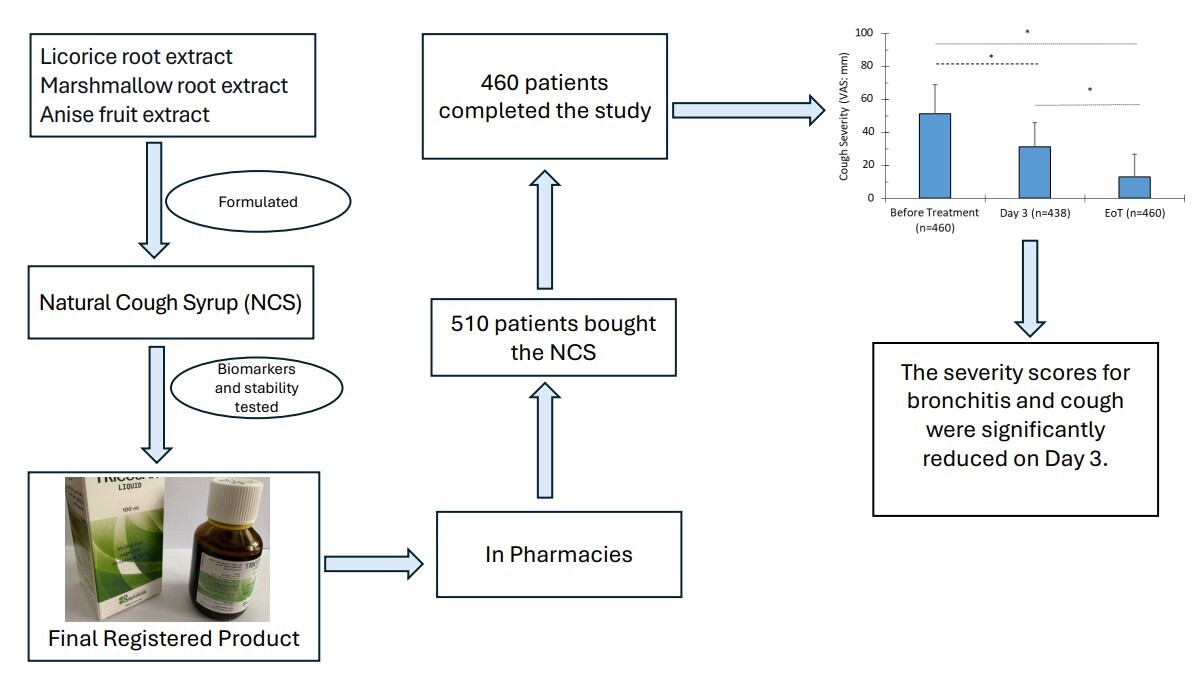
1. INTRODUCTION
Acute cough due to upper respiratory infection is the most common and bothersome symptom. Although cough is a self-limiting condition, it can have a disruptive effect and may persist for an extended duration, prompting patients to seek medical attention, miss work, and experience reduced productivity (Lee et al., 2013). Therefore, treating cough is essential, whether using a medicinal or over-the-counter product. In either case, cough treatment should focus on suppressing the symptom, reducing bronchiole inflammation, and eliminating the causative agent. Since an estimated 90% of upper respiratory tract infections are virally induced, antibiotics are not indicated and should be avoided to help prevent antibiotic resistance (Abdelmalek & Mousa, 2022; Fahey & Howie, 2001).
Treating virally-induced respiratory infection and its symptoms, including cough, requires multiple agents to target the disease and its symptoms. For instance, regulating the inflammatory process, strengthening specific immune pathways, and reducing inflammatory-related and unrelated symptoms are the key factors for quick and fast relief of viral infections and their symptoms. It has been shown that polyphenols and specifically combinations of specific flavonoids have all these multitarget effects that help reduce virally-induced respiratory infections (Mansoor et al., 2018; M. Butnariu, 2023).
Numerous natural products have been formulated as syrups, drops, tablets, capsules, lozenges, suppositories, or inhalations and are used as anti-cough remedies. These products contain either a single agent or a combination of agents and have demonstrated efficacy in randomized, placebo-controlled clinical trials (Kardos et al., 2021; Kemmerich et al., 2011; Kruttschnitt et al., 2020). Although the market for antitussive agents is broad, the development of a natural product with uniquely formulated ingredients that target cough-related mechanisms and reduce inflammation remains warranted and may provide greater benefits than currently available options.
Licorice root (Glycyrrhiza glabra L.), marshmallow root (Althaea officinalis L.), and anise fruit (Pimpinella anisum L.) extracts all contain fundamental flavonoids, polyphenols, and non-flavonoid compounds that may be effective against upper respiratory tract infections and their cough-induced effects. Marshmallow root extract has been used to treat irritative dry cough (Fink et al., 2018). It has been shown that extracts of A. officinalis L. inhibit rat tracheobronchial smooth muscle contractions (Alani et al., 2015). Furthermore, marshmallow root extract can protect inflamed mucosa by producing anti-inflammatory and antioxidative effects on activated proinflammatory macrophages and improving the migratory capacity of vascular endothelial cells (Bonaterra et al., 2020, 2022). A complex group of mucilaginous polysaccharides is thought to be responsible for coating the irritated mucosa in the mouth and throat, thus alleviating cough (Fink et al., 2018). However, studies have shown that bioactive low molecular weight compounds, such as flavonoid-O-sulfoglycosides, exhibit anti-inflammatory actions, including modulation of cytokine signaling, leukocyte migration, and the formation and regulation of the extracellular matrix in mucosal tissue (Sendker et al., 2017). Furthermore, A. officinalis contains the following flavonoids and phenolic compounds: hypolaetin-8-glucoside, isoquercitrin, kaempferol, caffeic acid, p-coumaric acid, ferulic acid, and p-hydroxybenzoic acid, all of which have anti-inflammatory and antioxidative effects that may be beneficial in respiratory infections (Al-Snafi, 2013; Gudej, 1991).
Licorice has been used as a cough suppressant in clinical settings (Asl & Hosseinzadeh, 2008; Shergis et al., 2015). The World Health Organization recognizes licorice as an expectorant for cough, bronchial mucus, and sore throat (Xiaoying et al., 2017). Licorice contains approximately 300 flavonoid compounds, with the root extract containing four major flavonoids: isoliquiritigenin, liquiritigenin, lichalocone, and glabridin (Wahab et al., 2021). Licorice roots, extracts, and active ingredients such as isoflavonoids, flavonoids, and glycyrrhizic acid have demonstrated efficacy in regulating respiratory functions, immunoregulation, and anti-inflammatory effects (Wahab et al., 2021). For example, liquiritin, liquiritin apioside, and liquiritigenin are the major antitussive compounds of licorice (Kuang et al., 2018). Furthermore, Wei et al. showed that liquiritin apioside attenuated the laryngeal chemoreflex in rat pups (Wei et al., 2020). Most recently, it has been proposed that licorice could be effective against COVID-19–induced inflammatory damage (Bisht et al., 2022).
P. anisum L. (Anise) extract analysis revealed several flavonoids, including catechin, naringin, apigenin, coumarin, rutin, quercetin, and kaempferol, as well as phenolic compounds such as chlorogenic acid, gallic acid, and the essential oil anethole (AlBalawi et al., 2023; Spinozzi et al., 2023). Anise has traditionally been used to relieve cough, respiratory congestion, gastrointestinal distress, and premenstrual syndrome (Shojaii & Abdollahi Fard, 2012; Singletary, 2022). Furthermore, P. anisum L. extract and its essential oil possess anti-inflammatory and antioxidative properties, are beneficial in wound healing (Ghlissi et al., 2020; Iannarelli et al., 2018), and have an analgesic effect (Tas, 2009).
Based on the bioactive flavonoids, polyphenols, and other active constituents of licorice, marshmallow, and anise extracts, a natural cough syrup (NCS) has been formulated and registered as an over-the-counter product to relieve dry and wet cough symptoms. In the present study, we aimed to present real-life and semi-qualitative data on the efficacy of reducing bronchitis severity score (BSS) (Kardos et al., 2014a; 2014b) and visual analog scale (VAS) assessing cough severity (Birring & Spinou, 2015) within a few days and to evaluate the tolerability of the NCS product in a prospective, open-label, uncontrolled, multicenter, pharmacy-based, observational study.
2. METHODS AND PARTICIPANTS
2.1. Natural cough syrup (NCS)
The NCS, TRICOSAN liquid, is registered in Saudi Arabia (No. 2019-1435-03). The batch number used for this study is 2206011, and it was manufactured by Bavarian Naturweg, GmbH, Germany, in June 2022. Each 100 mL of NCS contains three main active ingredients: licorice root extract (DER 3–4:1) (2.5 g) (EPO Istituto Farmochimico Fitoterapico S.R.L., Milan), marshmallow root extract (DER 4:1) (2.0 g) (EPO Istituto Farmochimico Fitoterapico S.R.L., Milan), and anise fruit extract (DER 4:1) (2% anethole) (1.0 g) (EPO Istituto Farmochimico Fitoterapico S.R.L., Milan). The combination was selected based on the above-mentioned information and favorable traditional herbal drug combinations of licorice root, marshmallow root, and anise fruit cited in the Commission E monographs (Blumenthal, 2000). The dried extracts were fully soluble in the liquid formulation. According to ICH guidelines, stability was studied and assured throughout the shelf life. The product was formulated as an expectorant to relieve symptoms of respiratory tract infections associated with cough.
2.2. Biomarkers determination and measurement of polyphenols and flavonoids in NCS
Glycyrrhizic acid was measured in the final product as a marker for licorice using a reverse-phase high-performance liquid chromatography (HPLC) method with a photodiode array detector, as described elsewhere (De et al., 2012). Asparagine, used as a marker for marshmallow, was determined by HPLC with a fluorescence detector (Plata-Guerrero et al., 2009), and the essential oil trans-anethole, used as a marker for anise, was measured by HPLC with UV-Vis detection (Fagundes et al., 2014).
The total flavonoid and polyphenol concentrations in the NCS were measured using UV spectroscopy methods. The total flavonoids method followed the European Pharmacopeia procedure, using oxalic acid, boric acid, and apigenin as standards. Total polyphenols were measured using a modified Folin-Ciocalteau reagent with pyrogallol as the standard (Blainski et al., 2013).
2.3. Study design, setting, and participants
The study was designed as a prospective, open-label, uncontrolled, multicenter, pharmacy-based, observational study. A total of 120 pharmacies in Saudi Arabia (Dammam and Riyadh) participated in the study. The study started in July 2022 and ended in December 2022. Pharmacists asked each participant who either had a prescription for TRICOSAN or requested cough syrup (since it is an over-the-counter product) if they would like to enroll in the study. However, participants’ decisions to purchase TRICOSAN and pharmacists’ consultations were independent of study participation. Pharmacists advised each participant to use a precise dose of TRICOSAN (5.0 mL using the auxiliary measuring cup) ter in die after meals. They also explained the proper procedure for completing and returning the questionnaire before, during, and after the therapy period. Participants returned the completed questionnaires in sealed envelopes to the pharmacies, which then delivered them unopened to the investigation committee.
Patients were asked to complete the questionnaire before starting TRICOSAN, after three days of use, and at the end of treatment (7–10 days) (EoT). If a participant took TRICOSAN for less than 7–10 days, the questionnaire was mailed and considered as the EoT assessment.
The eligibility criteria included purchasing TRICOSAN for acute cough due to upper respiratory tract infection, being at least 16 years old, willingness to follow the pharmacist’s recommendation, completing the questionnaire, and mailing it. Patients with hypertension, a history of allergies to Umbelliferae plants or any ingredient of TRICOSAN, and pregnant or breastfeeding females were excluded.
Since this study was observational and involved a registered product in Saudi Arabia, it was exempt from ethical committee review and informed consent requirements. Each participant returned a completed questionnaire, thereby consenting to participate. The study was conducted without collecting any participant identifiers, such as name or date of birth, ensuring anonymity. Additionally, pharmacists had no access to the completed questionnaires.
2.4. Bronchitis severity scores and visual analog scale
The persistence of symptoms, bronchitis severity, cough severity, associated symptoms, and duration of use were reported. The questionnaire was based on two validated methods: bronchitis severity score (BSS) (Kardos et al., 2014a; 2014b) and visual analog scale (VAS) for assessing cough severity (Birring & Spinou, 2015).
For the BSS, participants provided information about the severity of their cough, rattle, sputum, dyspnea, and chest pain during coughing. Each symptom was evaluated on a 5-point Likert scale ranging from zero (e.g., no cough or sputum) to four (most intense cough or sputum production). The total score of all symptoms ranged from 0 to 20, with 20 indicating the highest symptom intensity. TRICOSAN’s effect was evaluated by comparing BSS scores to baseline.
The VAS scale, representing cough severity, ranged from 0 mm (no cough) to 100 mm (very strong cough). The minimum clinically relevant change in VAS was defined as 17 mm (Lee et al., 2013).
2.5. Overall efficacy evaluation
Participants were finally asked to provide an overall evaluation of the efficacy of using NCS by reporting a 5-point Likert score ranging from 1 to 5, where 1 represents insufficient, 2 poor, 3 good, 4 very good, and 5 excellent efficacy.
2.6. Statistical analysis
The BSS and VAS data were reported as mean (± standard deviation), while some data were reported as frequencies. Differences in mean BSS and VAS scores across treatment time points (baseline, Day 3, and EoT) were analyzed using one-way ANOVA followed by Tukey’s HSD test. A P value of <0.05 was considered significant.
3. RESULTS
3.1. Percentage of Flavonoids in NCS and Biomarkers Concentrations
Based on the supplier’s certificate of analysis for each extract, the percentages of flavonoid and polyphenol content are presented in Table 1. The percentage of flavonoid content per dried extract ranged from 6% to 14%. Furthermore, the analysis of the natural cough syrup biomarkers and their concentrations in a 100 mL volume are shown in Table 2. Each biomarker was analyzed using HPLC according to the European Pharmacopoeia. The total flavonoids and polyphenols in the NCS were measured at 370 mg and 1810 mg per 100 mL of NCS, respectively (Table 2).
Table 1
Percentages of flavonoids and polyphenols in the plant extract constituents of the Natural Cough Syrup (NCS).
Table 2
Description of the Natural Cough Syrup, biomarker concentrations, and total polyphenols and flavonoids content.
3.2. Participants
Five hundred and ten participants were initially enrolled in the study, with 42.5% females and 57.5% males, ranging in age from 16 to 63 years (Table 3). However, 90% (460 participants) completed the study and returned the questionnaire. More than half (56%) of the completers were males, most were between 21 to 30 and 31 to 40 years old, and only 16% were smokers. The mean age of participants was 33.7 years. Of the 460 participants, 63% had cough symptoms lasting 3 to 4 days (Table 3). The mean baseline BSS was below the medium severity threshold of 10, whereas the VAS score was above 50 mm (Table 3).
Table 3
Participants’ demographics, symptoms, and baseline cough characteristics.
3.2. NCS reduced the Bronchitis Severity Score (BSS)
More than half of the participants (56%) used the NCS for 3 to 4 days (Figure 1). On Day 3 of NCS intake, the total BSS significantly decreased by 38.8% (P < 0.0001) (Table 4). Furthermore, at the EoT, the mean BSS dropped by 73% (P < 0.0001). These EoT levels were also significantly lower than those observed on Day 3 of treatment (55.8%; P < 0.0001) (Figure 1).
Figure 1
. Bronchitis Severity Score (BSS) at baseline (before treatment), Day 3, and end of treatment (EoT) presented as mean ± SD. P < 0.0001 (one-way ANOVA with Tukey’s HSD post hoc test). The number of participants at each time point is indicated in parentheses. The number of responses on Day 3 was lower compared to baseline and EoT.
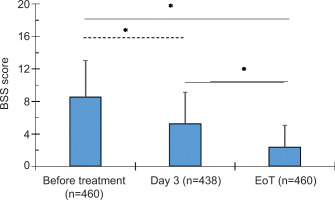
Table 4
Bronchitis Severity Scores (BSS) and Cough Severity Scores (VAS) during treatment with the Natural Cough Syrup (NCS).
The strongest symptoms at baseline were cough and sputum production, which decreased by 61% and 67%, respectively, at the EoT. Furthermore, rattle, chest pain during coughing, and dyspnea symptoms dropped by 80%, 85%, and 80% at the EoT, respectively.
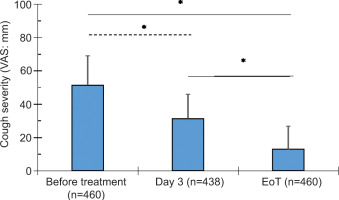
3.3. NCS reduced Cough Severity Score (VAS)
Following treatment with the NCS, the cough severity score (VAS) decreased by 39% (P < 0.0001) on Day 3 and by 75% (P < 0.0001) at EoT (Figure 2, Table 4). These reductions represent a 2- to 4-fold improvement on Day 3 and at EoT compared to the clinically relevant 17% cutoff.
Figure 2
. Cough severity measured by Visual Analog Scale (VAS, mm) at baseline (before treatment), Day 3, and end of treatment (EoT), shown as mean ± SD. P < 0.0001 (one-way ANOVA with Tukey’s HSD post hoc test). Participant numbers at each time point are indicated in parentheses. The number of responses on Day 3 was lower than at baseline and EoT.
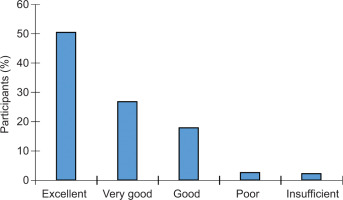
3.4. Participants’ efficacy satisfaction
Participants reported overall satisfaction with the efficacy of the NCS, with scores ranging from excellent to insufficient. Half of the participants rated the efficacy as excellent, 27% as very good, and 18% as good (Figure 3). The remaining 5% rated the product as having poor or insufficient efficacy.
3.5. NSC effect compared with placebo from controlled clinical trials
We compared the BSS results from the present study to placebo groups using BSS data from clinical studies. In this study, there was a 3.3-point decrease (39%) in BSS on Day 3 and a 6.2-point decrease (73%) at the EoT (7 to 10 days of treatment) (Table 5). In a double-blind, placebo-controlled randomized clinical trial with patients having a baseline BSS of 8.3, the placebo group showed a decrease of 2.3 points (28%) on Day 4 and 5.0 points (60%) at the EoT (Day 10) (Kemmerich et al., 2006). Another study reported a baseline placebo BSS of 8.6 (n = 109), which decreased by 2.5 points (29%) on Day 3 and by 5.3 points (62%) on Day 7 (Matthys & Heger, 2007). In a randomized placebo-controlled trial, placebo BSS scores (n = 189) were 7.4 at baseline, dropping to 6.8 (8%) on Day 3 and 4.4 (41%) on Day 7 (Albrecht et al., 2023). A meta-analysis of two double-blind, randomized, placebo-controlled trials reported an average placebo BSS change (n = 162) of 24% on Day 3 and 56% on Day 7 (EoT) (Völp et al., 2022). These comparisons indicate that the NCS used in the present study is more effective and reduces cough symptoms faster than the placebo effect (Table 5).
Table 5
Comparison of the NCS with placebo-treated patients in reducing BSS from controlled clinical trials.
| Studies | Treatment | N | Mean Baseline BSS | Percent Mean of BSS During Days of Treatment | |||
|---|---|---|---|---|---|---|---|
| Day 3 | Day 4 | Day 7 | Day 10 | ||||
| Kemmerich et al., 2006 | Placebo | 179 | 8.3 | — | 24% | — | 60% |
| Matthys & Heger, 2007Gillissen et al. 2013 | Placebo Placebo | 109 211 | 8.6 8.5 | 29% — | — — | 62% 35% | — 58% |
| Kahler et al. 2019 | Placebo | 127 | 8.2 | — | — | 39% | 48% |
| Albrecht et al. 2023 | Placebo | 189 | 7.4 | 8% | — | 41% | 68% |
| Current study | NCS | 460 | 8.5 | 39% | — | 73% | |
4. DISCUSSION
Almost 95% of patients using the NCS were satisfied with its efficacy, as their cough and related symptoms measured by BSS and VAS significantly decreased on Day 3 and further at the EoT. This study was designed to generate participant-reported outcomes following the acquisition of NCS from pharmacies. Compared with clinical trials using BSS as a primary endpoint, the percentage reduction in BSS in the present study was greater and faster than the placebo effect in relieving cough symptoms. These findings illustrate the efficacy of licorice, marshmallow, and anise extracts as an effective anti-cough remedy.
Coughing is a complex mechanism initiated by vagal afferent nerves mediated by C-fibers, rapidly adapting receptors, slowly adapting receptors, cough receptors, trigeminal afferent nerves, and esophageal nociceptors (Canning, 2010). Many stimuli may trigger one or multiple pathways. For example, in upper respiratory tract infection, inflammatory mediators such as prostaglandin E2 (PGE2) and bradykinin are expressed in higher amounts, initiating cough by activating afferent nerves via C-fibers. Additionally, the release of bronchoconstrictors during the inflammatory reaction (such as histamine and leukotrienes) induces mucus secretion, which activates rapidly adapting receptors, trigeminal afferent nerves, and esophageal nociceptors. Cough receptors, on the other hand, are activated by particles or acids (Canning, 2010). Therefore, reducing inflammatory mediators and mucus production, as well as suppressing the laryngeal chemoreflex, are likely to reduce coughing.
The NCS contains licorice, marshmallow, and anise extracts, each demonstrating cough-suppressing effects through different mechanisms. Licorice, in particular, exhibits anti-inflammatory activities by reducing prostaglandin E2 (PGE2), metalloproteinases, tumor necrosis factor-α (TNF-α), and free radicals, consistent with its traditional use in relieving cough, clearing phlegm, and alleviating pain (Yang et al., 2017). Licorice flavonoids are the primary contributors to these effects, modulating endogenous metabolites such as linoleic acid, sphingosine, tryptophanamide, N-linoleoyl taurine, corticosterone, L-acetylcarnitine, and leukotriene B4 via arachidonic acid, sphingolipid, and steroid hormone metabolism (X. Yu et al., 2019). Isoliquiritigenin, a major flavonoid in licorice roots, exhibits anti-inflammatory and antioxidant properties by regulating NF-κB and Nrf2 transcription factors and signaling pathways, offering protection in inflammation-induced pulmonary models (D. Yu et al., 2018). Glabridin, a significant isoflavone, exerts anti-allergic inflammatory effects by lowering serum IgE levels and improving respiratory function, while activating calcium-activated potassium channels and downregulating L-arginine-nitric oxide and cyclooxygenase (COX) production (Dogan et al., 2020; Parlar et al., 2020). Liquiritin and liquiritin apioside suppress the laryngeal chemoreflex by targeting laryngeal sensory fibers, underpinning their antitussive effects (Kuang et al., 2018; Wei et al., 2020). Among licorice polyphenols, glycerol reduces COX-2 expression and inflammatory cytokines IL-1β and IL-6 in stimulated macrophages (Shin et al., 2008), while glycyrrhizin, the main licorice constituent, inhibits the COX pathway and diminishes IL-6, TNF-α, and other inflammatory mediators, thereby exerting anti-inflammatory and analgesic activities (Wahab et al., 2021; Wang et al., 2015).
Marshmallow root extract (Althaea officinalis L.) has traditionally been used to treat irritative dry cough (Fink et al., 2018). It has been shown to reduce smooth muscle contraction through mechanisms independent of β-adrenergic receptors (Alani et al., 2015), and its cough-suppressing effects are partly mediated via 5-hydroxytryptamine 2 serotonergic receptors, which counteract C-fiber activation (Šutovská et al., 2009; Canning, 2010). The polysaccharides in marshmallow extract protect and soothe the irritated throat mucosa by reinforcing the natural mucus layer and supporting mucosal healing (Völp et al., 2022; Bonaterra et al., 2020, 2022). Additionally, the flavonoids and polyphenols in A. officinalis contribute significantly to its anti-inflammatory effects by modulating cytokine signaling, leukocyte migration, and extracellular matrix regulation (Sendker et al., 2017). Major flavonoids such as kaempferol, apigenin, flavonoid-O-sulfoglycosides, isoquercitrin, and quercitrin, along with polyphenols like caffeic acid, p-coumaric acid, and ferulic acid, have been identified in marshmallow root (Al-Snafi, 2013; Farhat et al., 2022; Gudej, 1991). Notably, quercitrin and isoquercitrin exhibit broad-spectrum antiviral activities against respiratory viruses including influenza and coronaviruses and possess immunomodulatory properties (Mbikay & Chrétien, 2022). Kaempferol has been shown in guinea pig models to reduce chronic airway inflammation by decreasing IL-5, IL-13, GM-CSF levels, eosinophil counts in bronchoalveolar lavage fluid, and transforming growth factor-β1 in lung tissue, while normalizing cough reflex sensitivity (Molitorisova et al., 2021). Caffeic acid, one of the most abundant polyphenols in marshmallow root extract, targets COX-2, inhibits prostaglandin E2 production, suppresses inflammatory cytokines such as IL-8 and IL-1, and exhibits strong antioxidant activity, reducing oxidative damage (Zielińska et al., 2021).
The major flavonoid constituents of Pimpinella anisum (anise) fruit extracts include catechin, quercetin, quercetin glycoside (rutin), naringin, apigenin, and, to a lesser extent, kaempferol, alongside phenolic compounds such as caffeic acid, p-coumaric acid, chlorogenic acid, and rosmarinic acid (AlBalawi et al., 2023; Spinozzi et al., 2023). As previously discussed, these flavonoids and phenolic compounds possess broad-spectrum antiviral and immunomodulatory activities that help reduce inflammation and mucus production, as well as suppress the laryngeal chemoreflex, thereby contributing to cough reduction (AlBalawi et al., 2023; Farhat et al., 2022; Mbikay & Chrétien, 2022; Molitorisova et al., 2021; Sun et al., 2019; Zielińska et al., 2021). Notably, anise also exhibits expectorant properties (Sun et al., 2019) and analgesic and antianxiety effects, which are important in the sedation of cough (Gudej, 1991).
The clinical part of the present study has limitations. First, it lacks a placebo-control group; second, it is a questionnaire-based observational study. Although the placebo effect can be significant, the efficacy of the NCS compared to placebo from published reports is evident (Table 5). Furthermore, since the outcomes are based on patients’ responses, the study reflects a real-world, unbiased observational design (Kardos et al., 2021). Additionally, the high response rate of 90% may suggest that participants were satisfied with the NCS’s efficacy and motivated to provide positive feedback.
5.CONCLUSION
The present study demonstrated the efficacy and safety of a syrup containing high levels of flavonoids and polyphenols, formulated from licorice, marshmallow, and anise extracts, in reducing coughing and cough-related symptoms in patients with acute cough within a shorter period than the typical self-resolving course. Additionally, the NCS was well tolerated.
Ethics approval and consent to participate
Since this study is an observational study on a product registered in the Saudi Arabia market, the study was exempted from the GCP Department of the Clinical Trial Administrations in Saudi Arabia Food and Drug Authority from ethics committee review and informed consent requirements. The study was carried out in accordance with the Declaration of Helsinki and with the guidelines and regulations of Saudi Arabia Food and Drug Authority. Each participant returned the filled questionnaire consented to participate.
Consent for publication
This is not applicable since the study was conducted anonymously and without patient identification, such as name or date of birth. Also, pharmacists had no access to any completed questionnaires.
Acknowledgements
Authors are grateful to all pharmacists and patients who participated in this project. The financial support of Herbresearch is acknowledged.
Conflict of Interest
Herbresearch Germany is the study sponsor but had no role in the experimental design of the study; in the collection, analyses, or interpretation of data. FQ is the scientific manager of the company. All other authors declare no conflict of interest.
Authors’ contributions
K.A.M., F.Q. and R.L. contributed to the study conception and study design. R.A. performed the investigation. K.Z.M. and F.Q. did the data curation and statistical analysis. K.Z.M. and F.Q. wrote the original draft. K.A.M. reviewed the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.