HISTORY OF RADIOPROTECTOR DEVELOPMENT
The harmful effects of ionizing radiation was discovered ever since the discovery of X-rays by Becquerel in 1896 but the extent of damage was not very clear (Radvanyi & Villain, 2017). The damaging effects of ionizing radiation were more pronounced after the atomic bomb attack in Hiroshima and Nagasaki, Japan in 1945. Since then, the incidence of nuclear terrorism has increased a lot. All these incidences created a global awareness and need to develop a suitable radioprotector. First attempt in the development of radioprotector was made by Patt and his co-workers. They found that the pre-treatment with naturally occurring amino acid cysteine increased the percentage of survival in mice and rats lethally irradiated with X-rays (Patt, Tyree, Straube, & Smith, 1949). However, it was not very successful in clinical trial for human application. Thereafter, several other compounds have been screened and evaluated for their efficacy in rendering radioprotection. However, none of them met the criteria for an ideal radioprotector as of yet. Only Amifostine, a compound created by the Walter Reed Army Research Institute, has been approved by the US Food and Drug Administration (USFDA) for human use (Kuruba & Gollapalli, 2018). However, owing of its high toxicity and inability to provide post-irradiation protection, the use of amifostine is restricted and provided under close medical supervision. Hence the application of amifostine is restricted to radiotherapy only to confer protection to normal cells. Recently, Neupogen and Neulasta have also been approved by United States Food and Drug Administration (USFDA) to treat hematopoietic acute radiation syndromes (H-ARS) (Cheema et al., 2018).
BACKGROUND
Radiation protection is the major area of concern in modern world. Ionizing radiations have wide range of applications in agriculture, industries and medical fields. In industries, radiations are frequently used in modification of polymers, polishing of gemstones, waste treatment, tyre industries, and in food industries to improve the shelf life and reduce contamination in foodstuffs (Chmielewski & Mohammad, 2005). In agriculture, it is used to reduce pathogenic microbes, increase juice yield, delay sprouting, improve re-hydration etc. (Kuan, Bhat, Patras, & Karim, 2013). Ionizing radiations are being used in a variety of medical applications, including radiotherapy to kill cancerous cells, in vivo imaging (CT scans, MRIs, ultrasounds, and X-rays) to diagnose disorders, and sterilisation of medical devices (Baskar, Lee, Yeo, & Yeoh, 2012; Gupta, 2013). Apart from that, it is frequently used in military, radiobiology, nuclear technology and space exploration (Moulder, 2002). Despite its significance, exposure to ionizing radiation has always been deleterious to human health. In present scenario, there are many chances for both planned and unplanned ionizing radiation exposure. Planned exposure includes medical imaging, radiotherapy and first responders etc. whereas unplanned exposures may happen during nuclear warfare, terrorist attack and many more scenarios. All of these events have raised the awareness for the development of a suitable radioprotector that can significantly prevent the damage induced by ionizing radiations. Hence the development of proper and safe radioprotector is an area of intense research in the scientific community. Amifostin is the only drug available to be administered as a radioprotector but still under medical supervision because of its side effects (Singh & Seed, 2017). Recently, Neupogen and Neulasta have also been approved by United States Food and Drug Administration (USFDA) to treat hematopoietic acute radiation syndromes (H-ARS) (Cheema et al., 2018). Extensive efforts have been done since ages, however till date no ideal and safe radioprotector is available. Herbal compounds having anti-inflammatory, anti-oxidant, anti-microbial, immuno-modulatory, free radical scavenging and anti-stress properties may be selected as a possible radioprotector based on the existing knowledge of radiation induced damages caused to living system. Various plants have been explored for development of promising herbal radioprotectors with certain degree of success, at least in preclinical set ups. An ideal radioprotector should be less toxic, cost effective, orally administered, rapidly absorbed, posses a good dose reduction factor (DRF). Determination of DRF is the most reliable parameter to evaluate any radioprotector including herbal preparations or plant extracts. DRF determined at LD50/30 of mice is considered most important
DRF=LD 50/30 radiation dose with drug/ LD 50/30 radiation dose without drug.
Hematopoietic syndrome can result in death due to sufficient loss of hematopoietic stem cells, hemorrhage, anemia and infection. Signs and symptoms of Gastrointestinal (GI) syndrome are nausea, vomiting, loss of appetite and abdominal pain, diarrhea (Jagetia, 2007). Loss of crypts, shortening of villi, decreased citrulline level, bacterial translocation from intestinal tract, intestinal epithelial cell denudation, dehydration and weight loss ultimately lead to death due to GI syndrome. Cardiovascular collapses, sepsis, severe hemorrhage, fluctuation in electrolyte concentration likely contribute to multi-organ failure and death (Elliott et al., 2014) at still higher doses of radiation exposure. This review mainly focuses on radioprotective potential of herbal and plant extracts. The results obtained from in vitro and in vivo studies of several herbs such as Tinospora cordifolia (Guduchi), Phyllanthus niruri (Bhumiamla), Allium sativum (Garlic), Podophyllum hexandrum, Hippophae rhamnoides, Ocimum sanctum, Tinospora cordifolia, Rhodiola imbricate, Emblica officinalis, Centella asiatica, Curcuma longa, Piper longum, Mentha piperita, Aegle marmelos and Zingiber officinalis have shown protection against radiation induced deaths in preclinical models (Dowlath et al., 2021).
RADIATION INDUCED DAMAGE
Exposure to ionizing radiation induces several types of damage and sickness depending upon the dose and dose rate received. Majority of damages induced by ionizing radiation are indirect and are mediated through the generation of free radical and reactive oxygen species (ROS). Exposure of ionizing radiation induces radiolysis of water which generates reactive oxygen species like hydroxyl ion (OH-), superoxides (O2-), Hydrogen peroxides (H2O2), hydrogen radical (.H) which reacts with cellular components like nucleic acids, lipids, proteins and induces cellular damage and death. DNA is the primary and most important target of radiation. ROS generated by radiation can induce alteration in nucleotide bases, strand breakage (both single and double), cross linkage which ultimately results in chromosomal abnormalities, mutations and cancer. Degradation of proteins by radiation, results in the loss of the activity of several enzymes and also the formation of protein carbonyls (Lakshmi, Tilak, Adhikari, Devasagayam, & Janardhanan, 2005; Reisz, Bansal, Qian, Zhao, & Furdui, 2014). Membrane lipids are also very susceptible to damage which results in lipid peroxidation ultimately affecting major cellular activities. Depending on the radiation dose, all of these changes result in cellular damage, cell death, altered cell division and depletion of stem cell pool, major organ system failure like hematopoietic system, gastrointestinal system, reproductive system, central nervous system and ultimately death (Hosseinimehr, Zakaryaee, & Froughizadeh, 2006). Hematopoietic system is readily affected by radiation exposure which results in haemorrhage, depletion of bone marrow progenitor cells, increased chances of opportunistic infections and anaemia (Kumar et al., 2007) (Zhou & Mi, 2005). The salivary gland and small intestine are extremely radiosensitive in the gastrointestinal tract. Radiation can cause damage to the intestinal villi and crypt cells in the intestine (Gupta, Kainthola, Tiwari, & Agrawala, 2020). Changes in puberty, gonadotropin deficit, hyperprolactinemia, and infertility are all symptoms of it in the reproductive system. Ionizing radiation has a significant impact on the central nervous system. It causes brain tissue destruction, neuroinflammation, changes in motor function, behaviour and eventually numerous neurological diseases. Long-term consequences include lung fibrosis, cancer, and other life-threatening disorders. (Figure 1 ).
CRITERIA OF AN IDEAL RADIOPROTECTOR
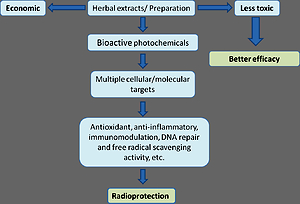
Thousands of chemical compounds and drugs have been evaluated for their radioprotective potential but most of them are still under preclinical trial. Most important prerequisite in the development of an ideal radioprotector is their minimal toxicity at the dose at which they are delivered. Besides this, it should have free radical scavenging ability, immunomodulation capability, anti-inflammatory, promote the DNA repair enzymes, upregulate antioxidant enzymes and enhance the recovery of hematopoietic and immune system (Figure 2 ). To meet these requirements a variety of immuno-modulatory agents, hematopoiesis stimulating factors, various chelating agents and growth factors have been screened. However, most of these agents showed a varied extent of protection and none of them are still applicable for human trial. Most importantly none of them have the ability to provide radiomitigation (post irradiation protection). We have categorized radioprotection into two types: Chemical or synthetic radioprotections and natural or herbal radioprotection.
SYNTHETIC RADIOPROTECTORS
Since last six decades, extensive efforts have been done in the area of radiation biology to develop synthetic radioprotector with minimal toxicity. Various compounds including cytoprotective agents, immuno-modulators, cytokines, vitamins and many DNA binding compounds have been evaluated for their radioprotective potential both in animal models and in vitro conditions (Haritwal, Gupta, Tiwari, Surve, & Agrawala, 2017). Synthetic sulfhydryl compounds like cysteine, mercaptoethylamine, cystine gained significant attention but their high toxicity and other side effects like nausea, vomiting, hypotension limited their use necessitating the search for other compounds with minimal toxicity and more acceptability (Maisin, 1989). Among all the sulfhydryl compounds, WR2721 (Amifostine) was found to be most effective and is currently used as adjuvant in radiotherapy (Singh & Seed, 2019). Certain immuno-modulator drugs and organometallic compounds were also screened. Immuno-modulators are non-cytokine compounds which facilitate the proliferation and differentiation of hematopoietic system susceptible to radiation induced damage. They have also been reported to suppress tumour growth in animal model. Application of gamma interferon (IFN-γ) enhances immune stimulation by activating T-cells and enhancing the expression of class-II major histocompatibility complex (MHC) (Castro, Cardoso, Gonçalves, Serre, & Oliveira, 2018). Few bacterial extracts, heat killed lactobacillus cells and protein associated polysaccharides have immunomodulatory effects (Taverniti & Guglielmetti, 2011). An immunomodulator, Ammonium trichloro-(dioxyethylene-O, O,) telluride (AS101) is under clinical trial for its application in treatment of cancer patients (Nair, Parida, & Nomura, 2001). 5-substituted-1,3,4-thiadiazole-2-thiols and 5-substituted-2-(3,4,5 trihydroxyphenyl)-1,3,4-oxadiazoles have shown wide range of potent antioxidant activities which can be investigated and evaluated for its radioprotection activity (Rabie, Tantawy, & Badr, 2018). Few cytokines such as IL-1, IL-6, TNF-α (Tumor necrosis factor-α) also show effective protection against lethal dose of radiation in animal model. Antioxidant enzymes mimicking compounds such as those of superoxide dismutase (SOD) with metals like Mn, Cu, and Fe at their active centres have also been developed (Borek, Ong, Mason, Donahue, & Biaglow, 1986; Nair et al., 2001; Vasilyeva, Bespalov, & Baranova, 2015). Certain nitroxides are also showing promising results in conferring radioprotection in animal model. Tempol is a nitroxide which is SOD mimetic and acts as free radical scavenger (Rosa, Corsi, Cavi, Bruni, & Dosio, 2021). Vitamin A, C, E, K being antioxidants can effectively scavenge free radical induced by ionizing radiation. Selenium along with vitamin E was found to be an effective free radical scavenger and enhancer of peroxide breakdown. Under the category of DNA binding ligands, Hoechst 33342 is the most studied radioprotector. It binds to the minor groove of DNA and induces protection by electron transfer ( Lubimova et al., 2001).
Most of the synthetic compounds studied so far exert their protective role by one of the following mechanism- i) Suppressing the generation of ROS.
ii) Enhancing DNA repair process.
iii) Upregulating free radical scavenging, or
iv) Reducing lipid peroxidation.
Although many of the screened synthetic compounds showed effective radioprotection in vitro and in vivo system but majority of them failed to show promising results in clinical trial for human application. Most significant drawback in using these synthetic compounds is their severe toxicity. Other limitations are high cost, inability to differentiate between normal cells and tumor cells (Demiral, Yerebakan, Simsir, & Alpsoy, 2002). They are also unable to provide post irradiation protection which is very essential in case of unplanned exposure like nuclear accidents, terrorist attack using nuclear warfare. Few possibilities to overcome these limitations are either chemical modification of these toxic compounds to relatively less toxic derivatives with same efficacy or to explore for other non- toxic alternative.
HERBAL RADIOPROTECTORS: SAFE ALTERNATIVE
Plants and herbal medicines are being used since ancient times for the treatment of many deadly diseases. Ayurvedic, Unani, Chinese, Japanese and Korean medicines rely on herbal preparations for the treatment of almost all diseases. Plants have inherent ability to tolerate environmental stress and sustain in varying environmental conditions. They are equipped with several enzymes and primary and secondary metabolites which favours their survival during environmental fluctuations. Some of these compounds have anti- inflammatory, immunostimulating, cytoprotective, antimicrobial, antioxidant ability which makes them important target for the development of radioprotector (Jagetia & Baliga, 2002; Maharwal, Samarth, & Saini, 2003; Parham et al., 2020). Some of the important factors favouring the development of herbal radioprotector are their less toxicity, easy availability, cost effectiveness. Many herbal formulations exhibits highly promising effects when delivered in case of deadly diseases like Parkison’s disease, Alzheimer’s diseases, Rheumatoid Arthritis, cancer and many other inflammatory diseases (Jena, Vikram, Tripathi, & Ramarao, 2010). This shows that plants and their formulations can relieve oxidative stress and thus can also be evaluated for their efficacy in providing protection against the damaging effects of ionizing radiation. Plants are very rich source of flavonoids such as orientin, catechin, vicenin, epigallocatechin. These flavonoids have potent antioxidant activity. Also many plant extracts contain carotene, ubiquinone, hydroubiquinone, polyphenols, fatty acids and certain amino acids which are having immunostimulating functions. Certain polyphenols, thiols, fatty acids, vitamins confer protection from carcinogenesis. Polyphenols, garlic extracts were also found effective in case of reducing radiation induced damage to hematopoietic system and central nervous system (Faramarzi, Piccolella, Manti, & Pacifico, 2021). These herbal preparations have been tested for their radioprotective efficacy as whole extracts, isolated constituents, fractionated formulations, polyherbal formulations (Paul, Unnikrishnan, & Nagappa, 2011)
Extracts of various herbs/ plants have been tested for their radioprotective activity viz: Podophyllum hexandrum, Hippophae rhamnoides, Ocimum sanctum, Tinospora cordifolia, Rhodiola imbricate, Emblica officinalis, Centella asiatica, Curcuma longa, Allium sativum, Piper longum, Mentha piperita, Aegle marmelos and Zingiber officinalis on different animal models. They have shown a promising radioprotection against variety of radiation induced damages.
The protection mediated by these natural plant extracts, their derivatives and some semi-natural compounds are through one or many of the following ways-
Free radical scavenging
Scavenging of free radicals is the most important mechanism of radiation protection induced by herbal radioprotectors. Free radical scavenging is mediated by several antioxidant enzymes present in cells which ultimately reduce the oxidative stress. Herbal extracts are known to contain a complex mixture of several polyphenols which could up-regulate mRNAs of antioxidant enzymes such as catalase, glutathione transferase, glutathione peroxidase, superoxide dismutase and thus may counteract the oxidative stress induced by ionizing radiations. For example, studies on Phyllanthus amarus shows that it enhances the activities of antioxidant enzymes including SOD, GSHPx, GR, GST in blood and tissues (Kumar & Kuttan, 2004). Tulsi, another important medicinal herb of Indian ethnic system, is widely studied for its radioprotective property. It contains two important flavonoids, orientin and vicenin which have been demonstrated for its radical scavenging activity (Devi, Ganasoundari, Vrinda, Srinivasan, & Unnikrishnan, 2000) . Some other herbal plants having rich polyphenolic content are Prunus Avium (Sweet Cherry) which is very rich in anthocyanin (Sisodia, Singh, Meghnani, & Srivastava, 2011). Mentha Piperita (Pudina), (Samarth & Samarth, 2009), leaves of palak (Spinacia Oleracea) are rich in carotenoid content (beta-carotene, lutein, Zeaxanthine), ascorbic acid, flavonoids and p-Coumaric acid (Bhatia & Jain, 2004). The polyphenolic compounds present in different herbal preparations helps to counteract the oxidative stress induced by ionizing radiation.
Decrease in lipid peroxidation
Free radicals generated by ionizing radiations oxidise cellular and membrane lipids resulting in lipid peroxidation (LPO). One of the most important criteria of an ideal radioprotector is the ability to inhibit lipid peroxidation and scavenge free radical. Majority of plant extracts and their formulations have been studied for their ability to inhibit lipid peroxidation. Extract of Tinospora cordifolia inhibited ferrous sulphate mediated lipid peroxidation (Kumar, Namita, & Goel, 2002). A wide variety of plants exert their radioprotective effect by decreasing LPO like Adhatoda vasica (Adulsa) (Kumar, Ram, Samarth, & Kumar, 2005), Amarantus paniculatus (Maharwal et al., 2003), P. hexandrum (Mittal et al., 2001) etc.
Anti-inflammation
A wide variety of plants and their extracts exert radioprotection through their anti- inflammatory activity. The flavonoids present in plants exhibit anti-inflammation activity either by stimulating the production of anti- inflammatory cytokines or by modulating radiation induced signal transduction pathway. They also inhibit the production of pro inflammatory cytokines such as IL-1, TNF-α, and IL-8. A number of plants, e.g. Tinospora cordifolia (Goel et al., 2004), Ginkgo biloba, Centella asiatica, Hippophae rhamnoides, Ocimum sanctum, Panax ginseng, Mentha arvensis, Syzygium cumini, Zingiber officinale (Jagetia, 2007) and some of their bioactive constituents such as quercetin, curcumin, c-phycocyanin, allicin, gingerol, caffeine exhibit anti-inflammatory properties.
Activation of antioxidant enzymes
The phytochemicals present in herbal plants up-regulate mRNA of antioxidant enzymes such as catalase, GSH transferase, GSHPx, superoxide dismutase (SOD) which is responsible for scavenging ROS ultimately reducing oxidative stress in cells induced by ionizing radiation. The polyphenols present in plants have antioxidant property which stimulates transcription of antioxidant genes (Lee, Lin, Yu, & Lee, 2017).
Protection and stimulation of hematopoietic system
A wide variety of plant products and their polyherbal formulations are found to be capable of recovering hematopoietic system following radiation damage. They enhance the regeneration of lymphoid and hematopoietic cells and thus minimize the chances of opportunistic infection after radiation injury. Ginkgo biloba, Podophyllum hexandrum, Spirulina sp., Tinospora cordifolia are some of the examples which show increased survival by enhancing hematopoietic recovery (Chen et al., 2004; Goel et al., 2004; Goel, Prakash, Ali, & Bala, 2007). Few polyherbal formulations like Triphala, Abana (Jagetia, Venkatesh, & Baliga, 2003) etc. are also evaluated for their ability to protect hematopoietic system. Similarly, Rhodiola sp. has many health promoting effects as well as it is being used to treat hernia, hysteria gastrointestinal ailments, infection, nervous disorder, head ache, post traumatic and vascular lesion of brain etc. It is also reported that Rhodiola has the potential to develop as radioprotective agent because of its enhanced hematopoiesis which leads to increase in whole body survival against lethal dose of radiation (Goel et al., 2006). These herbal formulations have potency to alter the hematopoietic damage by maintaining the higher number of hematopoietic stem cells, RBC count, haemoglobin, erythropoietin level. They also increases spleen Colony Forming Unit (CFUs) count and restore haematological parameters.
Protection to the GI system
A high radiation dose damages GI system by damaging the gastrointestinal barrier leading to the loss of water and electrolytes from the body (Hosseinimehr et al., 2006). Many of the plant extracts have successfully been found to confer protection against radiation induced GI damage. For example, extract of Podophyllum hexandrum has been reported to protect against radiation induced GI damage by increasing villi cellularity and survival of crypt cells by reducing apoptosis in crypt cells (Salin, Samanta, & Goel, 2001). Studies done on ginseng extract also shows reduced apoptosis in jejunal crypt cells and decrease in apoptotic cell death (Kim et al., 2001).
Protection of reproductive system
Reproductive system contains highly dividing cells. Exposure to ionizing radiation leads to the death of actively dividing cells. The impact of radiation exposure to testis and ovary leads increasing risk of passing on genetic diseases. Majority of the herbal plants and their extracts have the ability to reduce the radiation induced reproductive system damage. The efficacy of Podophyllum hexandrum extract to protect the male testicular system has been tested. Administration of Podophyllum hexandrum exhibited significant increases in testis weight, repopulation of the seminiferous tubules, increase in sperm count and mainly maintained stem-cell survival index. Reduction in the abnormalities of sperm morphology was also observed. The extract has also exhibited its ability to modulate antioxidant enzymes in the male reproductive system (Goel et al., 2002). Aqueous extract from leaves of Achillea millefolium L restores the reproductive organ weight, sperm count and sperm morphology as well (Dalsenter, Cavalcanti, Andrade, Araújo, & Marques, 2004). Extract of Xylopia aethiopica (Annonaceae) increases spermatozoa motility, sperm count, decrease in radiation induced sperm abnormality and restores the damage induced in seminiferous tubules (Adaramoye, Adedara, Popoola, & Farombi, 2010).
Decrease in DNA damage
Many of the medicinal plants have been evaluated for their ability to protect against radiation induced chromosomal aberrations and micronuclei formation which reflects DNA damages. For example, extract of pudina (Mentha Piperita) have shown significant decrease in micronuclei frequency in bone marrow of irradiated swiss albino mice (Rao & Rao, 2010). Similarly, treatment with leaf extract of Syzygium cumini (Jamun) also reduced micronuclei formation in human peripheral blood lymphocytes (Jagetia et al., 2002). Pre-treatment of tulsi (Ocimum sanctum), well known indian medicinal plant prevent clastogenesis in 2 Gy irradiated mice. Moreover, some clinical studies on small number of patients have also shown radioprotective activity of Tulsi. (Baliga, Rao, Rai, & Souza, 2016). Few other examples are Tinospora cordifolia (Guduc), Phyllanthus niruri (Bhumiamla), Allium sativum (Garlic) etc. All these plant extracts have inherent ability to protect against DNA damage and mortality caused due to exposure to ionizing radiation. The complex chemical constituents of these plants inhibit the action of several genes such as Protein Kinase C (PKC), Mitogen Activated Protein Kinase (MAPK) and many others leading to cell cycle arrest and facilitates repair of damage induced by ionizing radiations.
Activation of DNA repair process
Free radical production by radiation exposure leads to damage of genetic material which is the most severe damage induced by ionizing radiation. Herbal radioprotectors induce DNA repair by upregulating DNA repair genes and thus activating DNA repair enzymes (Kamran, Ranjan, Kaur, Sur, & Tandon, 2016). A wide variety of medicinal plants have been shown to activate DNA repair enzymes in irradiated mice such as mentha (Rao et al., 2010).
Plant extracts or their various formulations meet practically all of the parameters necessary for the development of an ideal radioprotector because herbal plant extracts exhibit their beneficial effects through multiple mechanisms (Table 1). Most importantly, these can be administered at the dose which is non-toxic and highly effective.
Table 1
Table represents various plant extracts and their biological activities which make them promising radioprotective agents.
S. No. | Name of plants | Test System | Maximum Tolerance Dose (MTD) | Uses and radioprotective efficacy | Reference |
1. | Hippophae rhamnoides | Rats, mice | Berries extract (40mg/kg body weight) Leaf extract (10-120 mg/kg Body weight) | Berries extract (RH1,2 and 3) ↑ whole body survival by acceleration of stem cell proliferation and immunostimulation. Whereas leaf extract (SBL-1) reduce GI damage by enhancing the proliferation of intestinal crypt cells and reducing chromosomal damage | (Bala, Gupta, Saini, Abdin, & Prasad, 2015; Bala, Prasad, Singh, Tiwari, & Sawhney, 2009; Goel et al., 2002; Kumar et al., 2002; Sureshbabu, Barik, Namita, & Kumar, 2008) |
2. | Podophyllum hexandrum | Mice | 200mg/kg body weight | Enhance survival by modulation of protein associated with cell death, enhance iron chelation in hematopoietic, GI and reproductive systems | (Goel, Prasad, Sharma, & Singh, 1998) (Kumar & Goel, 2000; Kumar et al., 2005; Kumar, Singh, Arora, Chawla, & Sharma, 2009; Mittal et al., 2001; Sagar et al., 2006; Salin et al., 2001; Samanta, Kannan, Bala, & Goel, 2004) |
3. | Ocimum sanctum | Mice | 6 g/kg | Antioxidant and anticarcinogenic activity, enhance survival by reducing oxidative stress by ↑ glutathione level | (Devi, 2001; Reshma, Ashalatha, Dinesh, & Vasudevan, 2005; Subramanian, Chintalwar, & Chattopadhyay, 2005; Tiwari et al., 2016) |
4. | Rhodiola imbricata | Swiss albino mouse | 1100-1300 mg/kg | Enhanced survival by enhanced proliferation of hematopoietic stem cells | |
5. | Allium sativum | Mice, Rats | 500 mg/kg (Oral) | Strengthening of anti - oxidant system | (Batcioglu et al., 2012; Katoch, Khan, Dwarakanath, & Agrawala, 2012; Tsubura, Lai, Kuwata, Uehara, & Yoshizawa, 2011) |
6. | Ginkgo biloba | Rats, Human | 0.11g/kg B.W. (E) 500μg/kg. (Human) | Attenuate radiation induced oxidative organ injury, Anticlastogenic, Antiapoptotic | (Emerit et al., 1995; Hannequin, Thibert, & Vaschalde, 1983; Khedr, Shafaa, Abdel-Ghaffar, & Saleh, 2018; Shin et al., 2009) |
7. | Tinospora cordifolia | HeLa cells, Mice | 10mg/kg (7 days prior to radiation) 75 mg/kg B.W. (testes) 200 mg/kg. B.W. | Metal chelation, enhance survival of mice against sublethal dose of radiation, radioprotective potential in testes, Reduce hematopoietic depression by increasing the level of growth factors | (Goel, Kumar, & Rana, 2002; Kumar et al., 2002; Pahadiya & Sharma, 2003; Rao et al., 2010; Sharma, Parmar, Verma, & Goyal, 2011; Singh, Tyagi, Rizvi, & Goel, 2007; Subramanian, Chintalwar, & Chattopadhyay, 2003) |
8. | Aegle marmelos | HPBLs and Mice | Bael fruit 2250 mg/kg (MTD) Leaf extract 6 g/kg B.W. (Oral route), 2.5 g/kg (i.p.) | Increase survival by reducing radiation induced sickness and organ damage, Reduce radiation induced DNA damage and genomic instability, Anticancer | (Baliga, Bhat, Pereira, Mathias, & Venkatesh, 2010; Dhankhar, Ruhil, Balhara, Dhankhar, & Chhillar, 2011; Jagetia et al., 2003; Jagetia, Venkatesh, & Baliga, 2004; Jagetia, Venkatesh, & Baliga, 2004) |
9. | Piper longum | Mice | 100 mg/kg (5 consecutive days) | Increase Glutathione level and reduce Alkaline phosphatase and lipid peroxidation in liver and serum | |
10. | Emblica officinalis | Rats and Mice | 3 g/kg (MTD) | Enhance Survival by recovery crypt cells and glutathione and lipid peroxidation in blood and GI , Antiulcerogenic activity | (Bhattacharya, Subramanian, Kamat, Bandyopadhyay, & Chattopadhyay, 2006; Jindal, Soyal, Sharma, & Goyal, 2009; Kb, Sabu, Lima, & Kuttan, 2004; Singh, Sharma, Nunia, & Goyal, 2005) |
11. | Centella asiatica | Rat and Mice | 500 mg/kg B.W. | Enhance survival by maintaining antioxidant enzyme level and prevent radiation induced behavioural changes | |
12. | Curcuma longa | Mice, Rats and Humans | 2000-8000 mg/day (MTD) | Inhibit acute and chronic effects of radiation, Reduce oxidative stress and provide protection to organs against radiation | (Aravindan, Madhusoodhanan, Ahmad, Johnson, & Herman, 2008; Goel & Aggarwal, 2010; Inano & Onoda, 2002; Jagetia, 2007; Nada, Hawas, Amin, Elnashar, & Elmageed, 2012) |
13. | Allium cepa |
| 40 mg/ml B.W. (E.D.) | Reduce radiation induced chromosomal damage, Modulate radiation induced changes in lipid peroxidation, glutathione, superoxide dismutation, Protect neuronal cells from oxidative stress | |
14. | Amaranthus paniculatus | Mice | 800 mg/kg (E. D.) | Enhanced survival by modifying glutathione and lipid peroxidation in liver and blood. | |
15. | Glycyrrhiza glabra | Mice, Rats and Humans | 100 μg/ml (E.D.) | Protect cellular DNA from radiation induced damage, microsomal membrane by reducing lipid peroxidation | |
16. | Mentha arvensis | Mice | 1000 mg/kg B.W. | Protection against radiation induced sickness and mortality | |
17. | Moringa oleiferia (Leaf Extract) | Mice, | 300 mg/kg B.W. (E D) | Ameliorate liver damage by decreasing radiation induced oxidative stress and chromosomal breaks in Bone marrow cells, Antitumor | (Bin-Meferij & El-Kott, 2015; Rao, Devi, & Kamath, 2001; Singh, Dayal, Cjha, & Mishra, 2015; Sinha, Das, Bhattacharjee, Majumdar, & Dey, 2011) |
18. | Zinziber officinalis | Mice | 300 mg/kg | Enhanced survival by reducing antioxidants and modulating the level of lipid peroxidation and glutathione | |
19. | Phyllanthus amarus | Mice | 750 mg/kg. (E.D.) | Protect Bone marrow and GI damage by reducing chromosomal damage and modulating the level of lipid peroxidation and glutathione | (KBN & Kuttan, 2007; Kumar et al., 2004; Londhe, Devasagayam, Foo, & Ghaskadbi, 2009) |
20. | Panax ginseng | Mice | 1200 mg/kg (Root extract) | Enhanced survival by improving radiation induced body weight loss, DNA damage and reducing crypt cells damage in GI, also reduced radiation induced infertility | (Kim et al., 1993; Kim et al., 2001; Kim et al., 2007; Kumar, Sharma, Saxena, & Kumar, 2003; Lee et al., 2006; Lee, Johnke, Allison, O'brien, & Dobbs, 2005; Pande, Kumar, & Kumar, 1998) |
All these features make them an attractive target for the development of safe and effective drug in comparison to the synthetic formulations. However for actual practical applicability of the herbal radioprotectors it is essential to have exact composition of the drug, mode of action of each component, knowledge of the side effects of any drug if present, pharmacokinetics of the drug inside the body, possibility of interaction between different components, interaction of different components of drug with different cellular components.
CONCLUSION
Development of radioprotector is very important requirement in present scenario. Although many synthetic drugs show effective radioprotection but practical applicability of these drugs is restricted owing to their high toxicity. The need to develop more effective and non-toxic alternative favours the development of herbal radioprotector. The complex chemical constituents of plants enable them to withstand and survive under harmful environmental radiations. This characteristic makes them important targets for the development as radioprotector. The efficacy of herbal extract largely depends upon its active constituents, the concentration of which is regulated by several factors. Some major factors include mode of extract preparation, climatic fluctuations, geographical location and the season of sample collection. All these factors result in variations in overall efficacy of herbal preparation. However, proper characterisation and scientific evaluation of herbal preparation may result in the development of an ideal radioprotector with minimal toxicity and highest efficacy.
Author contributions
TH and MT did the literature survey and compilation. PKA prepared the final draft and all authors approved.