INTRODUCTION
Palmitic or hexadecanoic acid (1) is one of the most plentiful and widespread saturated fatty acids present in almost all living creatures. It is a considerably weak natural monocarboxylic acid of the organic chemical formula CH3(CH2)14COOH, which has one carboxyl group and one straight long-chain pentadecyl group (aliphatic carbon chain) (PubChem, 2021). Historically, palmitic acid was first discovered by the famous French chemist Edmond Frémy in the year 1840 in saponified palm oil (palm oil is a vegetable oil extracted from the pulps of the fruits of oil palms) (PubChem, 2021). This acid is separated as a major component mainly from the palm oil, and it is also found in the two edible more-saturated vegetable oils palm kernel oil and coconut oil (PubChem, 2021). In biochemistry of humans, palmitic acid is almost the first fatty acid generated through fatty acid biosynthetic processes (Carta, Murru, Banni, & Manca, 2017; Kingsbury, Paul, Crossley, & Morgan, 1961). This renders it a principal body constituent in humans, since it makes up about 30% of human depot fat (Kingsbury et al., 1961). Similarly, palmitic acid can be considered as a major abundant lipid component of woman breast milk (Jensen, Hagerty, & McMahon, 1978). In human body, palmitic acid is the precursor to slightly-to-much longer fatty acids (Carta et al., 2017; Kingsbury et al., 1961). Excess palmitate and palmitic acid inhibit the activity and performance of acetyl-CoA carboxylase (ACC), the known biochemical enzyme which is accountable for synthesizing malonyl-CoA (which in turn is used to elongate the arising acyl chain) from acetyl-CoA, thus hindering extra palmitate production (Carta et al., 2017; Kingsbury et al., 1961). An important biological role of palmitic acid is its role in palmitoylation process (the process of addition of a palmitoyl group to some proteins to chemically modify them), which is very crucial for localization of many proteins in human cell membrane (Carta et al., 2017). It is worth mentioning that palmitoylation process is also very essential for the growth and replication of many infectious viruses (Zhang, Han, Osterrieder, & Veit, 2021). Industrially, palmitic acid and palmitates have many uses and applications as, e.g., surfactants (in soaps, cosmetics, detergents, pharmaceuticals, dairy products, etc.), natural additives (mainly to add texture, specially mouth feel, to processed foods; also in organic products), and gelling/thickening agents (mainly in military industries, since aluminum salts of both palmitic and naphthenic acids are the thickeners mixed with the volatile petrochemicals to synthesize napalm "note that the word napalm came from the names of the two acids, naphthenic acid and palmitic acid" which is an incendiary mixture/weapon/bomb or a flamethrower used in military actions) (Mba, Dumont, & Ngadi, 2015; Obibuzor, Okogbenin, & Abigor, 2012; Pike, 2021). Pharmacologically, palmitic acid and its esters/salts have many clinical applications in humans, e.g., retinyl palmitate ester is known to be a major source of vitamin A, ascorbyl palmitate compound is a fat-soluble ester form of the important water-soluble L-ascorbic acid "vitamin C" and it is also employed as an antioxidant food additive (approved with the E number E304), and paliperidone palmitate medication (known in the market as INVEGA Sustenna) is a long-acting antipsychotic agent used for the effective treatment of the severe psychological disease schizophrenia (this drug has been synthesized utilizing palmitic acid to generate the oily palmitate esteric form, thus to formulate the final drug product to act as a long-acting release carrier medium upon intramuscular injection to deliver long-acting depot form of this neuroleptic medication) (Health-Canada, 2021; M & Blaner, 2013; Nussbaum & Stroup, 2012).
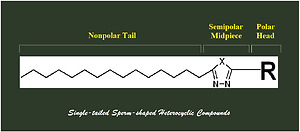
In organic, bioorganic, and medicinal chemistries, the specific simple and unique constructure of the fatty acid palmitic acid (Figure 1), which has a long open-chain 15-C aliphatic tail starts with an oxygenated head formed of one carboxyl group, gives it a very special snaky-carrier backbone of balanced amphiphilic characters for establishing new versatile intermediate/targeted organic molecules (e.g., nitrogenous heterocyclic compounds of expected antioxidant, antiinflammatory, antibacterial, antiviral, antifungal, antitumor, and immunomodulatory bioactivities) with pharmacokinetically-balanced properties and also for designing new medicinal structural formulas (of either new or old short-acting drugs) with needed special features of being long-acting and sustained-release medications (e.g., injected intramuscularly in depot forms). Single-tailed sperms have a very interesting characteristic swimmer-shaped carrier behavior which is required in several chemical and biological sciences. Through this current novel research, the dreams of multidisciplinary organic chemists to obtain human sperm-shaped heterocyclic molecules "Sperm-like Compounds" turned into achievable facts. In this new research paper, design, synthesis, elucidation, characterization, and possible application of four single-tailed oxygen/sulfur/nitrogen-containing heterocyclic derivatives of palmitic acid skeleton (Figure 2) were reported as the first discovery of this new class of unique sperm-like compounds of several nitrogenous heterocyclic aromatic nuclei (mainly, 1,3,4-oxa(thia)diazole and 1,2,4-triazole rings).
MATERIALS AND METHODS
Reactions were carried out with commercially obtainable reagents. All used chemicals (including solvents) were of very pure analytical grade "high quality". Microwave (MW) irradiation of MW reactions was performed in the laboratory automated MW synthesizer oven operated at a broad power range of 100-800 W/2.45 GHz. Thin-layer chromatography (TLC) was utilized to check the reactions progress (both conventional and MW) and the purity of all products, it was continuously performed on unmodified silica gel 60 F254 TLC aluminum plates of about 0.20 mm (E. Merck, Merck Millipore, Darmstadt, Germany) as the stationary phase, employing a solvent mixture of petroleum ether/ethyl acetate/absolute ethanol (6:3:2, v/v/v) as the eluent (using ultraviolet "UV" light wavelength of 254 nm to visualize and detect the resulted chromatogram spots). Evaporation/concentration purposes were done under reduced pressure in a rotary evaporator (rotavap). A lyophilizer "model FD8-8T" (purchased from SIM international, U.S.A.) was employed for the sake of the lyophilization process in the MW procedure. Melting points (M.P., °C) of all products were determined in special open glass capillaries using the common Fisher-Johns melting point apparatus (the obtained M.P. were uncorrected). IR spectra of the compounds 2a-2d were recorded and determined on Nicolet™ iS™ 10 Mid-Infrared FT-IR spectrometer (purchased from Thermo Fisher Scientific) (data expressed in υ in cm-1) using KBr disk at the Mansoura Faculty of Pharmacy Central Laboratory (Mansoura, Egypt) (for peaks, str. = strong, bro. = broad; for assignments, arom. = aromatic, aliph. = aliphatic). 1H NMR spectra of the compounds 2a-2d were registered on Varian Gemini-300 NMR spectrometer (Mercury-300BB "NMR300") at 300 MHz using the internal standard tetramethylsilane (TMS) at the Cairo Faculty of Science Microanalytical Center (Cairo, Egypt), and the chemical shift values (δ) were expressed in ppm downfield from TMS at a temperature of 30 °C using either DMSO-d6 or CDCl3 as solvents (1H NMR data were clearly reported as peak multiplicities: s for singlet peak, t for triplet peak, and m for multiplet one). MS analyses of the compounds 2a-2d were carried out on Shimadzu QP-2010 Plus apparatus operated at 70 eV and data were expressed by m/z (relative intensity "rel. int." in %) at the Cairo Faculty of Science Microanalytical Center (Cairo, Egypt). Specific C/H/N elem. anal. of the products 2a-2d were carried out also at the Cairo Faculty of Science Microanalytical Center (Cairo, Egypt) (results were expressed in %). Galloyl hydrazide was synthesized/purified/characterized via the preceding preparation procedures of the literature (Rabie, 2020). The detailed information of synthesis and characterization of compounds 2a-2d are available in the Appendix A.
RESULTS AND DISCUSSION
In the present work, four sperm-like heterocyclic derivatives of 1 were perfectly synthesized for the first time in organic chemistry history using the general model of amphiphilic rational design (Figure 3), as outlined in the synthetic pathways of Figure 4 (Rabie, 2021), and with yields of very good/excellent amounts (87.0-99.4%). The first derivative of 1 is the 1,3,4-oxadiazole one (2a) which is synthesized by the direct oxidative cyclocondensation of the acid 1 with an equimolar amount of galloyl hydrazide (also known as 3,4,5-trihydroxybenzoic acid hydrazide, obtained through the literature original procedures of (Rabie, 2020)) in the presence of the known potent dehydrating/oxidizing agent phosphorus oxychloride (POCl3) as a catalyst. This reaction demanded 4 h of continuous traditional reflux warming or 4 min of 30-sec periods of intermittent MW irradiation to afford the compound 2a. Refluxingly heating 2a with hydrazine hydrate in n-butanol "butan-1-ol" for 8 h gave the 1,2,4-triazole derivative of 1 (2b, the second derivative of 1). Replacement of the one oxygen atom of the formed heterocyclic 1,3,4-oxadiazole ring of compound 2a by a one analogous sulfur atom, via the interaction with thiourea in the versatile solvent THF by gentle heating in a well-sealed tube for about 8 h, gave the first 1,3,4-thiadiazole derivative of 1 (2c, the third derivative of 1 in general). The fourth/last derivative of 1 is the second 1,3,4-thiadiazole derivative (2d) which is synthesized, exactly like 2a, by the forthright dehydrative condensation of the acid 1 but with thiosemicarbazide (in a molar ratio of 1:1) in the presence of POCl3. This reaction proceeded with the same conditions as for 2a, as it required also 4 h of continuous conventional reflux heating or 4 min of 30-sec periods of MW irradiation to afford the compound 2d.
Unquestionable chemical characterization of the structures of the four targeted compounds 2a-2d was achieved using almost all spectroscopic (IR, 1H NMR, and MS) and major elemental analyses. In IR spectra, the disappearance of the well-known strong sharp distinctive peak of the C=O moiety of the hydrazide (i.e., of the galloyl hydrazide) was a very good marker for the hydrazide moiety transformation (with the carboxyl moiety of 1) to the closed heterocycle 1,3,4-oxadiazole (together with the existence of the distinctive IR peaks of the N-N/C=N/C-O moieties that compose the 1,3,4-oxadiazole nucleus, which also affirmed the 1,3,4-oxadiazole moiety formation) in 2a. Furthermore, the existence of the very characteristic broad and strong peak of the O-H group at a frequency of about 3452 cm-1 was an excellent marker of the presence of the three phenolic hydroxyl groups linked to the aromatic benzene ring (its presence and connection to C5 of the 1,3,4-oxadiazole nucleus was, in general, affirmed by the existence of the common featured peaks of the aromatic C=C/=C-H moieties). Finally, the observed strong peak representing the open chain of the repeated aliphatic C-H moieties (that is attached to C2 of the 1,3,4-oxadiazole ring), of the remaining original skeleton of 1, at a frequency of about 2909 cm-1 was a further affirmation of the structure of 2a. For 2b, the lack of the distinct IR peaks of the C-O moieties together with the existence of the IR peaks of the N-N/C-N moieties (which compose the heterocyclic 1,2,4-triazole ring) affirmed the 1,3,4-oxadiazole ring conversion to the corresponding 1,2,4-triazole ring (the presence of the 1,2,4-triazole ring primary NH2 group attached to N4 was proved by the two N-H stretches appearance at frequencies of 3269 and 3197 cm-1, respectively, which differ from that of the O-H stretching in being weaker and sharper). In addition, the existence of the very common distinct strong/broad peak of the O-H group at a frequency of about 3461 cm-1 was a valuable indication of the presence of the three phenolic hydroxyl groups linked to the aromatic benzene nucleus (its presence along with its attachment to C5 of the constituted 1,2,4-triazole nucleus was generally affirmed by the clear presence of the IR peaks of the phenyl C=C/=C-H moieties). Finally, the observed strong absorption peak representing the open chain of the repeated aliphatic C-H moieties (that is attached to C3 of the 1,2,4-triazole ring) at a frequency of about 2901 cm-1 was a further proof of 2b structure. For 2c, the disappearance of the previously present peaks of the C-O moieties together with the existence of the IR peaks of the 1,3,4-thiadiazole ring N-N/C-N/C-S moieties proved the transformation of the 1,3,4-oxadiazole nucleus to the corresponding 1,3,4-thiadiazole nucleus. Moreover, the existence of the very common strong/broad featured peak of the O-H group at a frequency of about 3465 cm-1 was a perfect proof of the presence of the three phenolic hydroxyl groups linked to the aromatic benzene ring (its presence along with its connection to C5 of the constituted heterocyclic 1,3,4-thiadiazole nucleus was generally proved by the existence of the common IR peaks of the phenyl C=C/=C-H moieties). Finally, the observed strong absorption peak representing the open chain of the repeated aliphatic C-H moieties (that is attached to C2 of the 1,3,4-thiadiazole rings) at a frequency of about 2913 cm-1 was a further confirmation of the chemical structure of 2c. Unlike the IR spectrum of 2c, the 2d spectrum displayed two strong N–H peaks at about 3273 and 3169 cm-1 as a result of the presence of the primary NH2 group linked to C5 of the heterocyclic 1,3,4-thiadiazole nucleus, while there was not any detected peak that could be assigned to a carboxylic OH group (proving the conversion/disappearance of 1).
Similarly, the 1H NMR observations largely supported the previous structural elucidations. The obvious disappearance of any distinct signal for the OH proton of a carboxyl group in the characteristic range of about 10.5-15.0 ppm (since the entire 1H NMR spectrum obtained from compound 2a analysis did not contain any signals at all in this wide region of the 1H NMR chart) was a perfect affirmation of the chemical transformation of the carboxyl group of the acid 1 (via the reaction with galloyl hydrazide) to the target substituted heterocyclic 1,3,4-oxadiazole nucleus. Moreover, the existence of the featured singlet signal at 5.39 ppm confirmed the presence of the three similar protons of the three neighboring phenolic hydroxyl groups which are linked to the phenyl ring (its presence/attachment to C5 of the constituted heterocyclic 1,3,4-oxadiazole nucleus was proved by the existence of the well-known distinct singlet signals of the benzene ring two protons at 6.79-7.31 ppm). The other varied signals, in the range of 0.84-2.51 ppm, representing all the protons of the 14 successive -CH2- moieties and one terminal -CH3 moiety of the saturated aliphatic pentadecyl group which is directly attached to C2 of the 1,3,4-oxadiazole ring were additional affirmations of the chemical structure of 2a. For 2b, the 1H NMR spectrum showed the same signals as for 2a and, in addition, it specifically displayed a singlet signal at 6.98 ppm which proved the presence of the two protons of the primary aromatic NH2 group linked to N4 of the heterocyclic 1,2,4-triazole nucleus. For 2c, the 1H NMR spectrum totally shows almost similar signals as those of the spectrum of 2a. For 2d, the clear disappearance of the very strong and broad featured signal of the carboxyl moiety OH proton of 1, the specific existence of a singlet signal at 6.99 ppm (which represents the presence of the two protons of the primary aromatic NH2 group which is linked to C5 of the heterocyclic 1,3,4-thiadizole nucleus), and the existence of several signals in the region of 0.82-2.53 ppm (representing all the protons of the 14 successive -CH2- moieties and one terminal -CH3 moiety of the fixed pentadecyl group that is directly attached to C2 of the 1,3,4-thiadiazole ring) are the main guides to elucidate and affirm its chemical structure. The resulted data of mass spectra and C/H/N elem. anal. of all the four newly-synthesized compounds 2a-2d were perfectly compliant with the targeted chemical structures.
Figure 3
A general amphiphilic model used for the rational design of the title zigzag/snaky compounds.

Figure 4
Synthetic pathways of sperm-shaped amphiphilic heterocyclic compounds (compounds 2a-2d) derived from natural palmitic acid.

In this model of amphiphilic structure (Figure 3), we can make use of this cocktail or hybrid pharmacophore in many different ways in pharmceutical and medicinal chemistry (Gao et al., 2021; Jubie et al., 2012; Mittal et al., 2021; Mushtaq et al., 2021; Rabie, 2021). First, pharmacokinetically, if we want to design certain drugs that have all the three main states of gradual polarity (polar, semipolar, and nonpolar) to effectively be distributed and pass through the diverse biological liquids and membranes, hundreds of compounds can be easily synthesized based on this backbone. Second, pharmacodynamically, several specific classes of medicines (as in many, e.g., antibacterial, antifungal, anti-SARS-CoV-2, antiviral, antimicrobial, antioxidant, anticancer, antidepressant, and antipsychotic drugs) should have certain considerable degrees of amphiphilic actions to successfully do their pharmacological mechanisms of action at the intended sites of action (where, conformationally, some biological targets of action need, for example, polar heads/moieties like amino/phenolic hydroxyl groups in the structures of the interacting molecules beside the less-polar and nonpolar moieties for adequate better effectiveness), thus the presented hybrid amphiphilic pharmacophore can provide an available suitable choice to design and synthesize compounds having these mixed lipophilic/hydrophilic properties or moieties in their structures. Third, in pharmaceutical formulation, some products and preparations need to be formulated using adequate amphiphilic carriers to pass through the diverse biological barriers of different polarities to reach certain deeper or protected targets of action; this can be effectively achieved through designing carriers having the newly-presented amphiphilic pharmacophore. Fourth, nutritionally, administration of such type of compounds/remedies which have amphiphilic structures is of additional health benefits for humans other than the therapeutic/formulational importance, e.g., the high biocompatibility of these molecules (owing to the natrual product, palmitic acid, from which they are constructed) with the biological systems will significantly reduce the expected side/adverse effects with improved bioavailability, and also these molecules will be considered as a good nutritional source of the important acid, palmitic acid (which will be released from these various derivatives upon metabolic transformation inside the human body), which is used inside the human body in many pivotal biological processes and for the building of important functional fats.
CONCLUSIONS
Palmitic acid is considered as the most prevalent naturally-occurring fatty acid with a swimmer or kite shape. It is, chemically, a saturated long-chain 16-carbon fatty acid with a snake-like scaffold. Herein in this research paper, I reported the succeeded design, synthesis, and physical/structural characterization of four sperm-like molecules of a newly-established class of single-tailed kite-shaped nitrogen-containing heterocyclic derivatives of palmitic acid for the first time, introducing a new discovery of this new single-tailed sperm-like pharmacophore which represents a novel and unique heteroaromatic ring system. These new unparalleled sperm-like compounds/agents are supposed to catch great interests from organic, bioorganic, and medicinal chemists because they are evident drug-like molecules and at the same time perfect drug carriers (from the pharmacokinetic point of view) with their gradual triple amphiphilic unrivaled constructure (which consists of a nonpolar long aliphatic carbonic chain, semipolar nitrogenous aromatic heterocyclic ring, and polar active trihydroxyphenyl or amino group) carrying extremely biologically active natural moieties (i.e., a palmitic acid tail or carrier swimming with one major midpiece of 1,3,4-oxadiazole/thiadiazole/triazole ring and a front head of hydrxyo/amino group(s)). The predominant high lipophilic nature of these palmitic acid anlogs is supposed to increase their expected antimicrobial activities (Jubie et al., 2012). This highly-balanced lipophilicity is also suggested to increase the permeability of the blood-brain barrier (BBB), leading to higher predicted antidepressant and antipsychotic effects of these analogs (Jubie et al., 2012). The current novel study will certainly pave the way for establishing a new class of organic/medicinal compounds "Sperm-shaped Drug-like Amphiphilic Molecules or SDAMs" which have pharmaceutical and pharmacological merits. The expected versatile therapeutic and medicinal applications of these palmitic acid derivatives need to be further extensively explored in the next few days.