1. INTRODUCTION
A wound is generally described as a disruption or discontinuity in the skin tissue. The process of wound healing is intricate and dynamic, requiring the synchronized involvement of various cell types, growth factors, and extracellular matrix elements. This process occurs in four interconnected phases: hemostasis, inflammation, proliferation, and remodeling (Singer et al., 1999; Broughton et al., 2006; Rhee et al., 2008). Initially, vasoconstriction and platelet aggregation occur to halt bleeding, forming a stable clot. This is followed by the recruitment of inflammatory cells, such as neutrophils, which release mediators and cytokines to promote angiogenesis, thrombosis, and re-epithelialization.
Fibroblasts then begin depositing extracellular matrix components, creating scaffolding for tissue repair. By days 5 to 7, fibroblasts start synthesizing new collagen and glycosaminoglycans, contributing to tissue strength and structure during the remodeling phase. This orchestrated sequence ensures efficient tissue repair and restoration of skin integrity (Midwood et al., 2004; Gurtner et al., 2008; Levenson et al., 1965). Siddha medicine takes a holistic approach to health, focusing on achieving harmony between the mind, body, and spirit. This traditional system heavily relies on the use of herbs, minerals, metals, and animal-derived substances in its treatments. Metals and minerals, while potentially harmful in excessive amounts, are meticulously purified and processed within the Siddha framework to ensure their safety for therapeutic use (Singer et al., 1999).
Ettikottai Mathirai (EM) is a polyherbal formulation traditionally prescribed for conditions such as 18 types of soolai (pain) and lumbar pain. Although Siddha practitioners have employed EM for wound healing, its efficacy lacks scientific validation. Notably, the formulation includes ingredients such as Milagu (Piper nigrum), Chukku (Zingiber officinale), Ettikottai (Strychnos nux-vomica), Kirampu (Syzygium aromaticum), and Elumichai (Citrus limon), all of which have demonstrated significant wound-healing properties in various scientific studies. Therefore, this study aimed to evaluate the wound-healing potential of Ettikottai Mathirai (EM) using the KB cell line.
2. MATERIALS AND METHODS
2.1. Choice of the test drug
The test drug “Ettikottai Mathirai” is one of the polyherbal formulations indicated for 18 types of soolai (throbbing pain), iduppu vali (sciatica), vaatham (related to joint disease), and pungal (wound), as described in the Siddha literature Naam Nadu Vaithiyam, written by Veeraperumal Pillai (Veeraperumalpillai, 2012).
2.2. Ingredients of Ettikottai Mathirai
The components of EM are presented in Table 1.
Table 1
Ingredients of Ettikottai Mathirai.
Purification of raw drugs
Chukku — Soaked in limestone water for 3 hours, dried, and the outer layer is peeled off (Murukesa Mudaliyar., 2013).
Milagu — Soaked in sore buttermilk for 1 to 1 hour 15 minutes and then fried (Murukesa Mudaliyar., 2013)
Kirampu — Bud of the flower was removed and then fired (Dhanik et al., 2017)
Ettikottai — Removed the outer skin and cotyledons of Ettikottai and deep fried it with ghee (Somasundaram, 2019).
2.4. Preparation of Ettikottai Mathirai
All the ingredients were roasted and made into a fine powder. The powder was gently ground by adding lemon juice until pills were formed (Veera Perumalpillai, 2012).
Dosage: 65-130mg/twice a day.
Adjuvant: Warm water or buttermilk.
2.5. In vitro wound healing activity of EM: An MTT assay-based evaluation
2.5.1. Cell line and Culture
A 96-well plate was seeded with KB cells at a density of 1 × 105 cells/mL and incubated at 37°C for 24 hours. After incubation, the medium was replaced, and cells were treated with different concentrations of the test sample, followed by an additional 24-hour incubation. After the treatment period, 10 µL of a 5 mg/mL MTT solution was added to each well, and the plate was incubated for 4 hours at 37°C. The supernatant was then removed, and 100 µL of DMSO was added to each well to dissolve the formazan crystals. The absorbance of the resulting solution was measured at 595 nm using a microplate reader (Jerard et al., 2020, Mosmann et al., 1983).
Using the following formula, the percentage of growth inhibition was determined:
2.5.2. In-vitro scratch wound healing assay
Cancer cells were seeded at a density of 2 × 105 cells/mL in 6-well plates and cultured for 48 hours or until confluence was reached. Next, using a sterile 200 µL micropipette tip, a linear wound was created in the monolayer. For twenty-four hours, cells were subjected to 110 µg/mL of test material. Following incubation, the cells were examined with an Olympus, Japan, inverted light microscope to take pictures (Dandannavar et al., 2019, Laura et al., 2004, Madhyaatha et al., 2013).
3. RESULTS & DISCUSSION
3.1. Evaluation of in-vitro cytotoxicity using the MTT assay
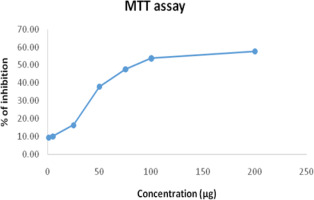
The cytotoxic impact of EM on KB cells at different concentrations is reflected in the cell viability percentages shown in Table 2. Figure 1 shows a dose-dependent inhibition of EM.
3.2. Wound healing scratch assay
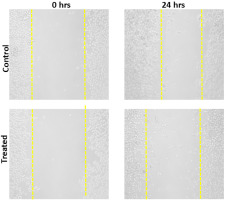
Microscopy images of the scratch assay on KB cells, showing cell migration at 0 and 24 hours for the Control group versus Ettikottai Mathirai treatment.
Wound healing is a critical health concern due to its implications for morbidity and mortality. The healing process involves distinct yet interdependent phases, including granulation, collagenation, collagen maturation, and scar maturation (Agarwal et al., 2009). The duration of healing largely depends on the rate of wound contraction, which accelerates wound closure by promoting rapid re-epithelialization. This process minimizes the migratory distance of keratinocytes, reducing the extracellular matrix needed to restore the damaged epithelium (Tang et al., 2007; Strodtbeck, 2001).
The wound healing (or scratch) assay is commonly used to measure two-dimensional cell migration. It involves creating an artificial gap in a confluent cell monolayer and monitoring cell movement under a microscope. As a simple and cost-effective method, the scratch test helps evaluate a drug's wound-healing potential. The assay specifically addresses the second stage of wound healing, characterized by keratinocyte and fibroblast migration and proliferation (Claark, 2013; Kaptaner et al., 2020; Martin, 1997). Keratinocytes play a pivotal role in re-epithelialization by migrating towards the wound site, proliferating, and forming an epithelial covering. This proliferation restores tissue integrity, aiding in the reconstruction of full-thickness skin. Growth factors such as TGF-α (transforming growth factor-α), KGF (keratinocyte growth factor), and EGF (epithelial growth factor) regulate keratinocyte proliferation, while integrins facilitate the re-epithelialization process (Schafer et al., 2007). As far as we are aware, this is the first report on EM and its wound healing activity. In addition to the wound healing experiment, we investigated the cytotoxic effects of Ettikottai Mathirai (EM) on the KB cell line. The MTT assay results demonstrated a dose-dependent cytotoxic effect, as indicated by the progressive reduction in optical density (OD) values with increasing EM concentrations. At lower concentrations (1–25 µg/mL), minimal inhibition was observed, reflecting a weak cytotoxic effect. However, significant inhibition was evident at 50 µg/mL, with a pronounced decrease in OD values at 75 µg/mL and higher. At 100–200 µg/mL, EM exhibited strong cytotoxicity, indicating its potential to suppress KB cell viability.
The primary objective of cancer chemotherapy is to selectively target cancer cells while minimizing damage to normal cells. The MTT assay confirmed that the inhibitory concentration (IC50) of EM was 128 µg/mL after 24 hours of treatment, highlighting its cytotoxic potential. To further assess its effects on cell proliferation and migration, a scratch assay was performed using KB cells treated with 128 µg/mL of EM. The assay involved creating a scratch in a confluent monolayer and monitoring cell movement in the presence and absence of drug treatment.
In the control group (without drug treatment), KB cells migrated into the scratched area within 24 hours, indicating normal cell motility. However, in the EM-treated group, the cells exhibited tattered morphologies and reduced migration, suggesting significant inhibition of cell movement. The scratch assay results indicate that EM at its IC50 concentration (128 µg/mL) disrupts cell adhesion and inhibits migration. Furthermore, the wound healing assay demonstrated that EM effectively suppressed KB cell migration compared to untreated controls, reinforcing its potential as an anti-metastatic agent.
Certain components in this formulation, including Milagu (Piper nigrum), Chukku (Zingiber officinale), Ettikottai (Strychnos nux-vomica), and Kirampu (Syzygium aromaticum), have been shown to exhibit wound healing properties. Alsareii et al. (2023) explored the wound-healing efficacy of a Milagu-containing bioactive hydrogel using an excision wound model in rats. Their findings demonstrated that the hydrogel significantly enhanced wound contraction, reduced inflammation, and promoted collagen deposition compared to placebo and control groups. The hydrogel’s effectiveness was attributed to piperine’s anti-inflammatory, antioxidant, and pro-collagen synthesis properties, suggesting its potential as a therapeutic agent for wound healing applications. Cheshfar et al. (2023) investigated the effects of Chukku (Zingiber officinale) extract ointment on pain and episiotomy wound healing in nulliparous women through a randomized clinical trial. The study found no statistically significant differences between the ginger and placebo groups in terms of pain and wound healing scores, but clinical improvements were observed. The authors suggested further research with different doses to evaluate its effectiveness. Tripathi et al. (2023) evaluated the wound-healing efficacy of the polyphenolic fraction of Etti (Strychnos nux-vomica) seeds using excision and incision wound models in rats. The study found that the extract significantly enhanced wound contraction, reduced epithelialization time, and improved tensile strength compared to the control. The polyphenolic content, including chlorogenic acid and caffeic acid, was suggested to contribute to its antioxidant and wound-healing properties. Muttaqien et al. (2024) additionally investigated the effects of Kirampu (Syzygium aromaticum) extract on wound healing, bacterial count, and IL-10 levels in methicillin-resistant Staphylococcus aureus (MRSA)-infected rats. The study found that clove flower extract significantly reduced bacterial count, lowered IL-10 levels, and accelerated wound healing time compared to the control group. The antibacterial and anti-inflammatory properties of the extract were attributed to its active compounds, such as eugenol, tannins, saponins, flavonoids, and alkaloids. Taken together, these studies highlight the potential of various bioactive compounds in promoting wound healing through different mechanisms.
4. CONCLUSION
In conclusion, Ettikottai Mathirai demonstrates a significant wound-healing effect on the KB cell line, promoting rapid wound closure and fibroblast formation. This effect is likely attributed to its potent anti-inflammatory and antioxidant properties. These findings provide a foundation for future research to explore the molecular mechanisms and signaling pathways involved in fibroblast proliferation and migration stimulated by Ettikottai Mathirai.
Acknowledgment
The authors acknowledge the support and facilities provided by the National Institute of Siddha, Tambaram sanatorium, and our Sathiyabama University of science and Technology, Chennai for their support in this study.