1. INTRODUCTION
The skin is continually exposed to microorganisms which is the primary barrier against environmental factors, rendering it susceptible to infections and dermatological conditions. Among bacterial pathogens linked to skin infections, Cutibacterium acnes (formerly Propionibacterium acnes) and Staphylococcus epidermidis are of particular significance (Dessinioti & Katsambas, 2024). C. acnes is a gram-positive anaerobic bacterium primarily associated with acne vulgaris, a prevalent dermatological condition affecting a substantial portion of the population (Mayslich et al., 2021). Conversely, S. epidermidis, a commensal bacterium, can transition into an opportunistic pathogen, leading to skin infections ranging from minor issues to life-threatening diseases (Severn & Horswill, 2023). These infections are often accompanied by oxidative stress and inflammation, which play crucial roles in the pathogenesis of skin disorders such as premature aging, impaired wound healing, and increased risk of skin cancer (Azzimonti et al., 2023; Fournière et al., 2020).
The rise of antibiotic-resistant strains and the side effects of conventional therapies have made managing skin infections increasingly challenging (Dessinioti & Katsambas, 2024). As a result, there is growing interest in exploring alternative therapeutic agents with antimicrobial, antioxidant, and anti-inflammatory properties to address skin-related conditions effectively. Plant-derived compounds, particularly polyphenols, are increasingly recognized for their multifaceted bioactivities and potential as safer alternatives to conventional treatments (Hano & Tungmunnithum, 2020). Among these, Etlingera plants (Zingiberaceae family), which are rich in phenolic compounds, are highly valued for their medicinal properties and economic importance in many Asian countries, including Indonesia, Thailand, and India (Koch et al., 2024). Etlingera pavieana (Pierre ex Gagnep) R.M.Sm, a species distributed in Thailand, is traditionally used for its medicinal properties, such as relieving fever and flatulence (Srisook & Srisook, 2019). Additionally, its aromatic properties, characterized by a unique flavor and fragrance, make its rhizome a popular local food ingredient and spice in Eastern Thailand (Koch et al., 2024; Srisook & Srisook, 2019). This distinctive aroma not only enhances its culinary appeal but also stimulates interest in its potential as an ingredient in skincare products, particularly in cosmetics, where fragrance plays a significant role. The growing consumer preference for natural fragrances in cosmetics further underscores the potential of E. pavieana as a valuable ingredient, given its unique aroma and bioactive properties.
While previous studies have reported the antimicrobial activity of various Etlingera species against a range of bacterial and fungal pathogens (Barbosa et al., 2020; de Sousa Ferreira et al., 2023; Naksang et al., 2020; Naksang & Rachtanapun, 2015; Tachai & Nuntawong, 2016; Ud-Daula & Mohammad, 2019), the antimicrobial efficacy of E. pavieana against skin pathogens remains underexplored.
This study aims to evaluate the antimicrobial, antioxidant, and anti-inflammatory activities of E. pavieana rhizome extract (EE) against C. acnes and S. epidermidis, two key skin pathogens. Additionally, the extract’s effects on biofilm formation and cell surface hydrophobicity, which are critical factors in bacterial adhesion and persistence, are investigated. The nontoxicity of the extract on dermal cells is also assessed to support its safety for topical use. By identifying the major phenolic compounds in the extract and testing their antibacterial activity, this study seeks to provide insights into the bioactive components contributing to its efficacy. The findings will contribute to the development of E. pavieana as a natural ingredient for dermatological applications, particularly in addressing microbial infections, oxidative stress, and inflammation in skin care.
2. MATERIALS AND METHODS
2.1. Plant extraction
The maceration extraction method was employed to obtain the EE extract from fresh rhizomes of E. pavieana (The Plant List Record 244738). The rhizomes were harvested from a local farm in Chantaburi province, Thailand, and authenticated by a plant taxonomist from the Department of Biology at Burapha University, Chonburi, Thailand. A voucher specimen (PI-SCBUU-001-2) was deposited at the Faculty of Science, Burapha University.
The rhizomes were cleaned, dried in a hot air oven, and ground into fine powder. The powdered rhizomes (300 g) were mixed with 3 L of food-grade ethanol (Union Chemical and Equipment Co. Ltd., Bangkok, Thailand) in a tightly sealed glass container and allowed to macerate for 5 days at room temperature, with intermittent shaking to ensure thorough extraction. Following maceration, the mixture was filtered using Whatman No. 1 filter paper (Millipore Sigma, MA, USA). The plant material underwent two additional extraction cycles to maximize yield. The combined filtrates were then concentrated using a rotary evaporator. The resulting viscous brown EE extract was freeze-dried, collected in a sterile tube, and stored at −20°C until further analysis.
The 4-methoxycinnamyl p-coumarate (MCC) was purified by the author following the protocol outlined in Srisook et al. (2017) and Iawsipo et al. (2018), while p-coumaric acid (PCA) was generously provided by Asst. Prof. Ekaruth Srisook from Burapha University (Srisook et al., 2017). The 1H-NMR spectrum for the MCC compound is provided in the supplementary data.
2.2. Total phenolic content analysis
The richness of phenols in the EE extract was analyzed using the standard Folin–Ciocalteu method. Briefly, 25 µL of extracts at concentrations ranging from 0.5 to 4 mg/mL were mixed with 30 µL of Folin–Ciocalteu reagent (Merck, Darmstadt, Germany) and 100 µL of deionized water. The mixture was incubated in the dark for 6 min, followed by the addition of 250 µL of 7% sodium carbonate (Na2CO3) and 195 µL of deionized water. The resulting mixtures were allowed to stand for 30 min at room temperature. Absorbance was measured at 765 nm using a microplate reader. The concentrations of total soluble phenolic compounds (TPC) were determined using a standard curve of aqueous gallic acid solutions and were expressed as mg gallic acid equivalents per gram of extract (mg GAE/g).
2.3. Bacterial strains
Clinical isolates of C. acnes (DMST14916) and S. epidermidis (DMST4369) were obtained from the culture collection of the Department of Medical Sciences, Ministry of Public Health, Thailand. C. acnes was cultured in brain heart infusion (BHI) medium supplemented with 1% glucose under anaerobic conditions for all tests. S. epidermidis was maintained in tryptic soy broth (TSB) under normal atmospheric conditions, except when Mueller–Hinton (MH) medium was specified in the methods. All media were purchased from HiMedia Laboratories Pvt. Ltd., Maharashtra, India.
2.4. Agar well diffusion assay
The antimicrobial activity of the EE extract was assessed using the agar well diffusion method. BHI agar supplemented with 1% glucose was used for C. acnes, while MH agar plates were used for S. epidermidis. Each agar plate was inoculated by evenly swabbing the surface with a sterile cotton swab dipped in the bacterial culture, ensuring uniform distribution.
Wells with a diameter of 6 mm were created in the agar. To each well, 50 µL of EE extract or pure compounds (MCC or PCA) at various concentrations, dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich Corp., MO, USA), were added. Wells loaded with clindamycin or ampicillin served as positive controls, while those with DMSO served as negative controls. The plates were then incubated at 37°C for 72 h under anaerobic conditions for C. acnes and for 18–24 h under aerobic conditions for S. epidermidis. After incubation, the diameters of the inhibition zones surrounding the wells were measured in millimeters (mm).
2.5. Minimum inhibitory concentration and minimum bactericidal concentration determination
The minimum inhibitory concentration (MIC) of the EE extract against C. acnes and S. epidermidis was determined using the agar dilution method following the Clinical and Laboratory Standards Institute (CLSI) guidelines. Initially, 4 µL of bacterial suspensions, adjusted to appropriate turbidity levels, were inoculated onto MH agar plates. Serial two-fold dilutions of the extract were prepared in DMSO and applied in 5 µL aliquots atop the bacterial inoculum. Once the extract was dried, the plates were incubated at 37°C for 72 h under anaerobic conditions for C. acnes and 24 h for S. epidermidis. After incubation, the plates were inspected for visible bacterial growth inhibition, and the MIC was determined as the lowest concentration of the extract at which no bacterial growth was observed.
The minimum bactericidal concentration (MBC) of the EE extract was determined by subculturing aliquots from the MIC plates onto new agar plates. DMSO treatment was used as a negative control. Subsequently, the plates were incubated at 37°C under suitable conditions for each bacterium as described previously. The MBC was defined as the lowest concentration of the extract that resulted in no visible bacterial growth on the subculture plates.
2.6. Assessment of biofilm formation inhibition
The biofilm-forming potential of C. acnes under EE extract treatment was determined using a method slightly modified from Gannesen et al. (2019). Briefly, a starter inoculum (OD600 = 1.8) was diluted in 1:15 with BHI medium. Then, 100 µL of the diluted inoculum was mixed with 50 µL of EE extract at various concentrations, resulting in final concentrations ranging from 0.08 to 2.67 µg/µL, and the mixture was added to a 96-well plate. The plates were incubated at 37°C for 72 h under anaerobic conditions. After incubation, planktonic cultures were carefully removed by pipetting, and wells were washed with phosphate-buffered saline (PBS, pH 7.4). Plates were dried in a laminar flow cabinet. Biofilms were fixed with absolute ethanol for 15 min, after which the ethanol was removed. Then, plates were dried before staining with 0.1% crystal violet for 15 min. Excess crystal violet was washed off with PBS. Biofilm-bound crystal violet was extracted with absolute ethanol for 30 min at room temperature, and absorbance at 570 nm was measured using a microplate reader. Experiments were performed in triplicate and repeated twice. Additionally, post-treatment bacterial numbers were assessed by conducting parallel experiments with the same treatment conditions and measuring OD600.
2.7. Evaluation of cell surface hydrophobicity
The effect of EE extract on bacterial cell surface adhesion to hydrocarbons was evaluated using the bacterial adhesion to hydrocarbon (BATH) assay, as modified from Lebeloane et al. (2024). Briefly, 1 mL of inoculum (OD600 = 1) in BHI medium was treated with 200 µL of EE extract and incubated at 37°C for 48 h. Following incubation, cells were resuspended in PBS buffer (pH 7.4) to an OD600 of 0.7–0.8 (A0) and mixed with 400 µL of toluene. The mixture was vortexed for 1 min and then incubated at 37°C for 15 min to allow phase separation. Absorbance of aqueous phase was subsequently measured at 600 nm (A1). The percentage of bacterial adherence to hydrocarbons (A) was calculated using Equation 1.
The mean results from duplicate experiments, repeated four times, were compared to the untreated control to assess the impact of EE extract on bacterial adhesion.
2.8. 2,2-Diphenyl-1-picrylhydrazyl scavenging assay
The antioxidant activity of the EE extract was assessed using the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging assay according to Khatun et al. (2021), with some modifications. Various concentrations of the extract (0.25, 0.5, 1, 2, and 4 µg/µL) were mixed with DPPH solution (0.2 mM in methanol) (MedChemExpress, NJ, USA), with ascorbic acid (MilliporeSigma, MA, USA) used as a positive reference. After incubation in the dark at room temperature for 20 min, absorbance was measured at 517 nm using a microplate reader. The percentage of DPPH scavenged by the extract was calculated using the following formula:
Where ADPPH is the absorbance of the control (DPPH solution without extract), and Asample is the absorbance of the sample (DPPH solution with extract).
Additionally, the EC50 value, which represents the concentration of the extract required to scavenge 50% of DPPH free radicals, was determined.
2.9. Bovine serum albumin denaturation assay
The anti-inflammatory activity of the EE extract was determined using the bovine serum albumin (BSA) denaturation assay adapted from Gunathilake et al. (2018). Various concentrations of the extract (0.03125–0.5 µg/µL) or ibuprofen (0.625–10 mM, as a positive control) were prepared in PBS. A mixture of 50 µL BSA solution (2% w/v in PBS) and 150 µL of the test solution was incubated at 37°C for 15 min and then heated at 70°C for 10 min. The reactions were performed in triplicate. After cooling, 150 µL of the mixture was transferred to a well of a 96-well plate, and absorbance was measured at 660 nm using a microplate reader. The percentage inhibition of BSA denaturation by the extract was calculated using the following formula:
Where Acontrol represents the absorbance of the control (BSA solution without extract), and Asample represents the absorbance of the sample (BSA solution with extract).
2.10. Cytotoxicity assessment
The cytotoxicity of the EE extract was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on primary human dermal fibroblasts (ATCC PCS-201-012TM), as previously described by Silva et al. (2020), with slight modifications. Cells were seeded in 96-well plates at a density of 1 × 104 cells per well and allowed to adhere for 24 h in a CO2 incubator under humidified atmosphere. Various concentrations of the EE extract in 0.3% DMSO (ranging from 50 to 400 µg/mL) were then added to the wells, and the cells were incubated for an additional 24 h. Following incubation, 10% MTT solution (AppliChem, Darmstadt, Germany) (5 mg/mL in PBS) was added to each well, and the plates were incubated for 4 h at 37°C in an incubator. The formazan crystals formed were dissolved in 100 µL of DMSO, and absorbance was measured at 570 nm using a microplate reader. Cell viability was expressed as a percentage relative to the untreated control, with 0.3% DMSO used as a negative control.
2.11. Quantitative HPLC analysis of phenolic compounds in the Etlingera pavieana extract
The quantitative analysis of phenolic compounds in the EE extract was performed using a validated HPLC method adapted from Srisook et al. (2021). Purified compounds previously identified in the plant served as reference standards (Srisook et al., 2017). An Agilent 1260 Infinity II HPLC system equipped with a UV-Vis diode array detector (DAD) was utilized for the analysis. Separation was achieved using a Phenomenex Luna C18 column (4.6 x 250 mm, 5 µm particle size). A mobile phase consisting of 100% water (solution A) and 100% methanol (solution B) was used with the following elution conditions: 0–15 min at 50% A and 50% B, 15–20 min at 20% A and 80% B, and 21–30 min at 50% A and 50% B, with a flow rate of 1.0 mL/min. Detection was performed at wavelengths of 254, 280, 310, and 320 nm, with the column temperature maintained at 35°C. 10 µL volume of each sample was injected, and chromatographic separation was conducted over a total run time of 30 min.
To quantify individual compounds in the EE extract, standard compounds were used to generate linear calibration curves by plotting peak areas against corresponding concentrations. Quantification was performed at the wavelengths that provided the strongest signal: MCA at 254 nm, PCA at 310 nm, and MCC and MCH at 320 nm. The chromatogram, however, was recorded at 280 nm to ensure all compound peaks were clearly visible. To confirm the accuracy of the quantification, a spiked sample containing 10 µg/mL of standards was also analyzed.
2.12. Statistical analysis
The data were presented as the mean ± standard deviation (SD) of at least six values. Graphical representations were constructed using Microsoft Excel 2021. To evaluate significant differences among groups, either a paired t-test or a one-way analysis of variance (ANOVA) was performed, followed by Tukey’s test using Minitab 18 software (Minitab Inc., PA, USA). Statistical significance was established at p < 0.05.
3. RESULTS
3.1. Etlingera pavieana extract and its total phenolic content
The ethanolic extract from E. pavieana (EE), obtained via maceration, displayed a viscous consistency, a brown coloration, and a distinctive anis-like aroma. The yield of the extract was 24.60 g, corresponding to an 8.2% yield. The richness of phenolics in the EE extract was confirmed using the Folin–Ciocalteu method, with gallic acid as the standard (y = 0.0016x + 0.0097; R2 = 0.9981). The total phenolic content (TPC) was found to be 33.11 ± 0.01 mg GAE/g.
3.2. Antimicrobial activity of the Etlingera pavieana extract against Cutibacterium acnes and Staphylococcus epidermidis
The antimicrobial efficacy of the EE extract against C. acnes and S. epidermidis was clearly evident in the agar well diffusion assay. The extract displayed significant inhibitory effects on bacterial growth, as evidenced by the clear inhibition zones around the wells containing various extract concentrations (Figures 1A and 1B). The inhibition zones increased dose-dependently, reaching 24.00 ± 1.73 mm for C. acnes and 15.67 ± 0.58 mm for S. epidermidis at 256 µg/µL (Table 1). This dose-dependent response emphasizes the potency of the EE extract as a natural antimicrobial agent against common bacterial pathogens implicated in skin-related conditions. Notably, the extract demonstrated particularly pronounced activity against C. acnes, suggesting potential therapeutic benefits for managing acne vulgaris.
Figure 1
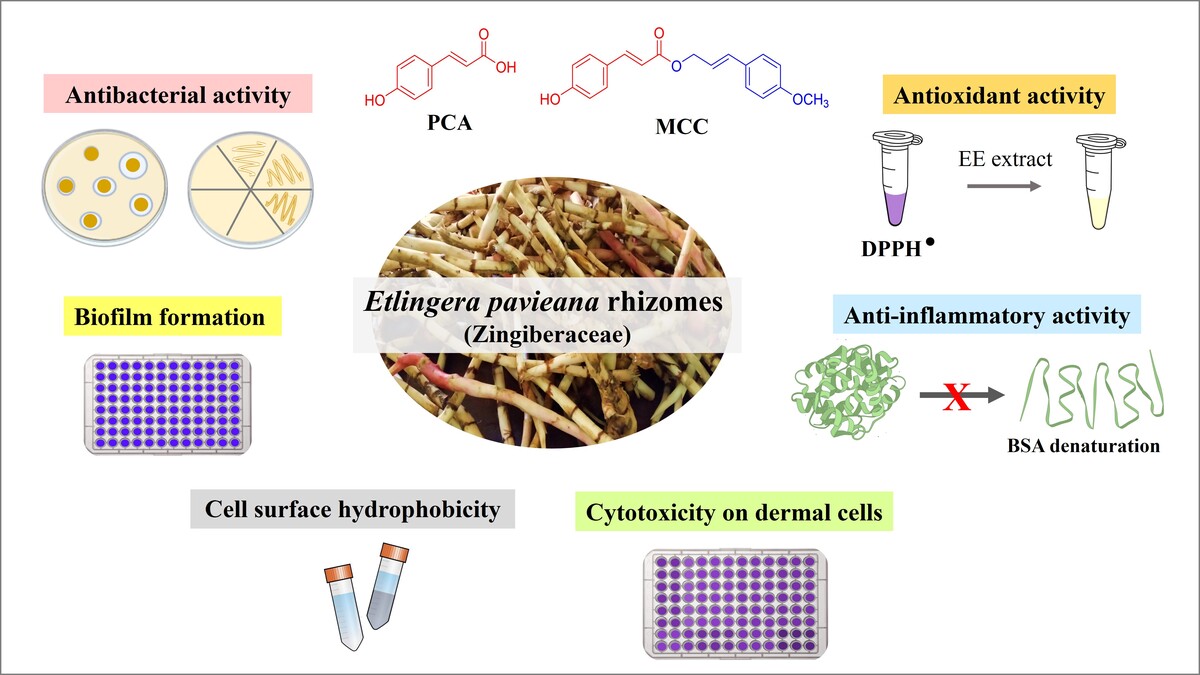
Antibacterial activity of EE extract and MIC/MBC determinations. Representative well diffusion assay plates showing the inhibitory effect of EE extract on C. acnes (A) and S. epidermidis (B) Representative plates for determining minimum inhibitory concentration (MIC) by agar dilution assay (C) and minimum bactericidal concentration (MBC) (D) for C. acnes.
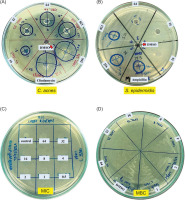
Table 1
Inhibition zone diameter (mm) of EE extract against skin-related bacteria.
The MIC values of the EE extract against C. acnes and S. epidermidis were determined using an agar dilution assay (Figures 1C and 1D). The MIC values were 4 µg/µL (0.02 mg/spot) for C. acnes and 64 µg/µL (0.32 mg/spot) for S. epidermidis (Table 2). The MBC of the EE extract was then determined by subculturing aliquots from the MIC plates onto fresh agar plates. The MBC values were 16 µg/µL (0.08 mg/spot) for C. acnes (Figure 1D) and 256 µg/µL (1.28 mg/spot) for S. epidermidis. The MBC/MIC ratio of 4 for both strains indicates a bactericidal effect (Patil & Patel, 2021). These findings further support the antimicrobial efficacy of the EE extract and its potential as a natural agent against bacterial infections.
3.3. Inhibition of biofilm formation and reduction in cell surface hydrophobicity by Etlingera pavieana extract
Given that C. acnes demonstrated a more pronounced response to EE extract compared to S. epidermidis in preliminary antimicrobial assessments, further experiments were focused exclusively on C. acnes. The objective was to elucidate the potential mechanisms underlying the antibacterial effects of EE extract, specifically targeting biofilm formation and cell surface hydrophobicity.
The effect of EE extract on biofilm formation by C. acnes was evaluated in conjunction with its impact on bacterial growth (OD600), using concentrations lower than the MIC (4 µg/µL). The results, illustrated in Figure 2A, indicate a significant reduction in biofilm formation starting at concentrations as low as 0.17 µg/µL, as evidenced by decreased absorbance values at A570 compared to the negative control. Additionally, approximately 50% biofilm formation was inhibited at a concentration of 0.67 µg/µL and higher. This reduction in biofilm formation was observed without a corresponding decrease in bacterial growth, highlighting the specific inhibitory effect of EE extract on biofilm production.
Figure 2
Inhibition of biofilm formation and reduction in cell surface hydrophobicity by EE extract in C. acnes. (A) Biofilm formation (A570) was quantified using crystal violet staining, alongside monitoring bacterial growth (OD600) after EE treatment for 72 h. Representative images of wells stained with crystal violet illustrate biofilm reduction at increasing EE concentrations. Images below depict bacterial aggregates in culture tubes, with visibly reduced aggregation upon EE exposure. (B) Cell surface hydrophobicity (%) of C. acnes was assessed using the BATH assay after exposure to various EE extract concentrations for 48 h. Representative images of solvent separation (toluene and water phases) show increased turbidity in the aqueous phase, indicating reduced hydrophobicity as EE concentration increases. Experiments were performed in replicates (A: n = 6, B: n =8), and the results are presented as Mean ± SD. Significant differences from the untreated control (Neg) are marked with an asterisk (*), at p < 0.05.
Furthermore, the effect of EE extract on cell surface hydrophobicity of C. acnes was assessed using the BATH assay. The results, presented in Figure 2B, showed a significant decrease in hydrophobicity at all tested concentrations of EE extract, starting from 0.25 µg/µL. The reduction in hydrophobicity likely precedes and contributes to the observed inhibition of biofilm formation, as decreased hydrophobicity impairs the initial bacterial adhesion necessary for biofilm development.
3.4. Antioxidant activity of the Etlingera pavieana extract determined by DPPH radical scavenging assay
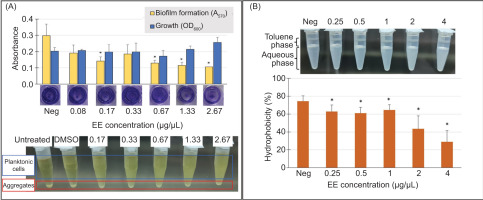
The EE extract exhibited antioxidant activity in the DPPH radical scavenging assay (Figure 3A). At concentrations ranging from 0.25 to 4 µg/µL, the extract exhibited a dose-dependent increase in DPPH radical scavenging activity, reaching a maximum of 55.51%. Although its EC50 value (3.68 ± 0.19 µg/µL) was higher than that of ascorbic acid (14.84 ± 0.22 ng/µL), these findings highlight the potential of the EE extract as a natural antioxidant for combating oxidative stress-related conditions.
Figure 3
Antioxidant and anti-inflammatory activities of EE extract. (A) DPPH radical scavenging activity of EE extract was assessed at various concentrations (0.25, 0.5, 1, 2, and 4 µg/µL). Ascorbic acid was used as a positive reference. Error bars represent the standard deviation of triplicate measurements from three experiments. (B) Concentration-dependent anti-inflammatory activity of the EE extract assessed using the BSA denaturation assay. Dark gray bars represent the inhibition of BSA denaturation (%) by EE extract at concentrations ranging from 0.03125 to 0.5 µg/µL, while red bars represent the inhibition by the positive control Ibuprofen at concentrations ranging from 0.625 to 10 mM. Different letters (A-D) denote significant differences within the same treatment at p < 0.05.
3.5. Anti-inflammatory activity of the Etlingera pavieana extract assessed by the BSA denaturation assay
In the BSA denaturation assay, the EE extract demonstrated a concentration-dependent inhibitory effect on the denaturation of BSA (Figure 3B). At the lowest concentration tested (0.03125 µg/µL), the extract exhibited a modest inhibition of 20.72%, with inhibition increasing gradually with higher concentrations. Notably, at concentrations 0.25 µg/µL, the EE extract reached its maximum inhibitory effect, nearly completely inhibiting BSA denaturation and providing protection nearing 100%. Similarly, the positive control Ibuprofen exhibited a concentration-dependent inhibitory effect on BSA denaturation. At lower concentrations (<2.5 mM), Ibuprofen exhibited relatively low inhibition percentages, less than 10%. However, at concentrations of 2.5 mM and above, Ibuprofen demonstrated substantial inhibition, ranging from 32.71 to 94.01%. These results reveal the potential of the EE extract to prevent protein denaturation, highlighting its promising role as an anti-inflammatory agent.
3.6. Cytotoxicity of the Etlingera pavieana extract on human dermal fibroblasts
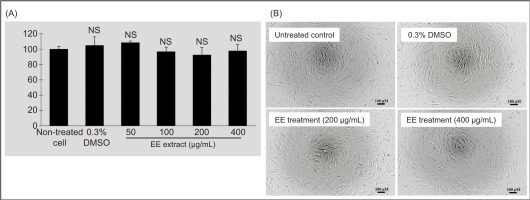
To ensure the safety of the EE extract, its cytotoxicity was evaluated on primary human dermal fibroblasts using the MTT assay. The results demonstrated that the EE extract did not adversely affect cell viability at concentrations ranging from 50 to 400 µg/mL (Figure 4). Cell viability remained above 90% across all tested concentrations, indicating that the EE extract is not detrimental to these dermal cells.
Figure 4
Cytotoxicity assessment of EE extract on primary human dermal fibroblasts using the MTT assay. Cells were treated with varying concentrations of the EE extract (50–400 µg/mL) for 24 h. (A) Cell viability was expressed as a percentage relative to the untreated control, with 0.3% DMSO serving as the solvent control. Error bars represent the standard deviation of triplicate measurements. NS denotes values not significantly different from non-treated cells (p <0.05). (B) The photographs were taken under inverted microscope at 20X objective lens magnification. The scale bar represents 100 µm. No significant morphological changes were observed in the treated cell groups compared to the control, supporting the conclusion that the extract is nontoxic to the tested cells.
3.7. Quantitative analysis of some phenolic compounds in the Etlingera pavieana extract using HPLC and their antibacterial activity
Four bioactive compounds, namely 4-methoxycinnamyl p-coumarate (MCC), trans-4-methoxycinnamaldehyde (MCA), 4-methoxycinnamyl alcohol (MCH), and p-coumaric acid (PCA), previously identified for their anti-inflammatory, anticancer, and antimicrobial properties in E. pavieana (Iawsipo et al., 2018; Poonasri et al., 2023; Srisook et al., 2017; Tachai & Nuntawong, 2016), were utilized as reference standards for the quantitative analysis of the EE extract. The HPLC chromatogram (Figure 5) displayed approximately 20 distinctive peaks, including four corresponding to the reference standards, two of which were predominant. MCC, the uncommon phenylpropanoids, exhibited the highest concentration at 3.67 ± 0.08 µg/mg, followed by PCA at 0.76 ± 0.03 µg/mg (Table 3).
Figure 5
HPLC chromatogram of EE extract and spiked EE extract detected at 280 nm. The chromatogram illustrates the separation of phenolic compounds in the EE extract (blue line) with four reference standards (dashed lines) in the background: 4-methoxycinnamyl p-coumarate (MCC), trans-4-methoxycinnamaldehyde (MCA), 4-methoxycinnamyl alcohol (MCH), and p-coumaric acid (PCA). The upper panel displays the chemical structures of these compounds. The middle panel shows the chromatogram of the EE extract alone, while the lower panel presents the chromatogram of the EE extract spiked with the four standards (10 µg/mL), highlighting the corresponding peaks for each compound.
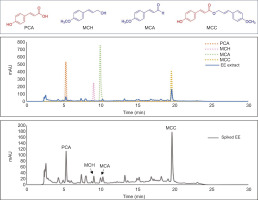
Table 3
Retention time (tR) and concentration of four bioactive compounds in Etlingera pavieana extract.
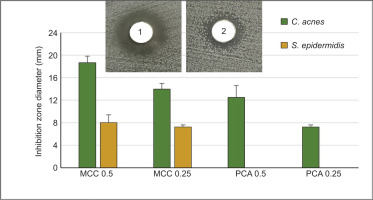
The antibacterial activity of MCC and PCA against C. acnes and S. epidermidis was further assessed using the well diffusion method. MCC exhibited significant antibacterial activity against C. acnes, with inhibition zones of 18.67 ± 1.15 mm at 0.5 mg/mL and 14 ± 1.00 mm at 0.25 mg/mL (Figure 6). In comparison, PCA showed smaller inhibition zones of 12.5 ± 2.12 mm at 0.5 mg/mL and 7.25 ± 0.35 mm at 0.25 mg/mL. For S. epidermidis, MCC displayed antibacterial activity, while PCA showed no detectable inhibition zones at either concentration. These findings highlight the superior antibacterial efficacy of MCC relative to PCA and strengthen their significant contribution to the overall antibacterial activity observed in the EE extract.
Figure 6
Antibacterial activity of 4-methoxycinnamyl p-coumarate (MCC) and p-coumaric acid (PCA) against C. acnes and S. epidermidis, as determined by the well diffusion method. The bar graph presents the inhibition zone diameters (in mm) of MCC and PCA at concentrations of 0.5 µg/µL and 0.25 µg/µL. Insets show representative images of the agar diffusion assay MCC at 0.5 µg/µL (1) and PCA at 0.5 µg/µL (2). ND indicates no detectable inhibition.
4. DISCUSSION
While the biological activities of E. pavieana have been widely studied, there is limited understanding of its efficacy against specific skin pathogens, particularly C. acnes and S. epidermidis. This study provides novel insights into the effectiveness of E. pavieana against these bacteria, as well as its antioxidant and anti-inflammatory properties. These multifaceted actions suggest promising therapeutic applications for E. pavieana in dermatological treatments, offering an effective strategy for addressing skin infections and their associated consequences.
The EE extract exhibited more potent antibacterial activity against C. acnes compared to S. epidermidis, indicating its potential utility in managing acne vulgaris, a condition primarily driven by C. acnes (Huang et al., 2022). Similar findings have been reported in extracts from other Etlingera species, which demonstrated stronger activity against C. acnes than Staphylococci (Nurul Saidah et al., 2019; Syafriana et al., 2021). This difference in efficacy may be attributed to variations in bacterial membrane composition or resistance mechanisms. Interestingly, the limited effect on S. epidermidis could be advantageous, as this bacterium plays a role in regulating C. acnes colonization and inflammation (Claudel et al., 2019; Huang et al., 2022).
To evaluate the antimicrobial effectiveness of the EE extract, the agar dilution method was chosen because it closely mimics natural growth environments, allowing for accurate visual assessments. While this method generally yields higher MIC values, it enables simultaneous testing across multiple samples. The MBC/MIC ratio of 4 observed for both C. acnes and S. epidermidis indicates that the EE extract exerts a bactericidal effect (Patil & Patel, 2021).
Biofilm formation is a critical factor in the persistence of C. acnes in skin infections and contributes to its resistance to conventional treatments (Coenye et al., 2021). Cell surface hydrophobicity, which affects microbial adherence, plays a key role in biofilm development (Danchik & Casadevall, 2021; Mirani et al., 2018). The EE extract inhibited biofilm formation, primarily at sub-MIC concentrations, without a significant reduction in bacterial growth. This antibiofilm activity, along with its ability to decrease cell surface hydrophobicity, suggests its potential to disrupt C. acnes adhesion and biofilm development, enhancing its therapeutic potential. Similar antibiofilm effects have also been reported for E. pavieana essential oil (Wongsariya et al., 2024).
Inflammatory mediators and oxidative stress are key contributors to the development of acne vulgaris and other skin disorders (Cruz et al., 2023; Dréno et al., 2020). Effective treatments aim to reduce C. acnes levels, inflammation, oxidative stress, and modulate the skin microbiome (Cruz et al., 2023; Mardani et al., 2021; Shan et al., 2022). The EE extract reduced BSA protein denaturation, indicating potential anti-inflammatory properties (Silvestrini & Silvestrini, 2023). Its dose-dependent antioxidant activity, shown through DPPH radical scavenging, is likely due to its phenolic compounds, as seen in other Etlingera extracts (Koch et al., 2024; Sinsuebpol et al., 2023; Wahyuni et al., 2021). While its antioxidant capacity was lower than ascorbic acid, this property complements its antimicrobial and anti-inflammatory activities, offering a comprehensive approach to treating acne.
A crucial property of any treatment is minimal side effects. The EE extract showed low cytotoxicity in MTT assays, with cell viability exceeding 90% at concentrations up to 400 µg/mL. This is consistent with previous studies indicating that E. pavieana rhizome extracts and essential oils have low toxicity in various cell lines and animal models (Chiranthanut et al., 2022; Wongsariya et al., 2024; Srisook et al., 2017). These results reinforce the extract’s potential for safe use in topical skincare formulations.
HPLC analysis identified MCC and PCA as the primary phenolic compounds in the EE extract. MCC, an ester formed by the conjugation of a methoxycinnamyl alcohol group with PCA, belongs to the larger family of PCA derivatives. Both compounds are known for their antimicrobial, antioxidant, and anti-inflammatory properties, which contribute to the extract’s bioactivity (Abazari et al., 2021; Iawsipo et al., 2018; Poonasri et al., 2023; Srisook et al., 2017; Tachai & Nuntawong, 2016). The bioactivities of E. pavieana extract observed in this study can be attributed to the structural features of its major phenolic compounds, MCC and PCA.
The polyphenolic nature of these compounds enables interactions with bacterial proteins and extracellular polymeric substances (EPS), disrupting biofilm formation. Specifically, MCC’s ester linkages and methoxy groups likely enhance its ability to penetrate bacterial membranes and form stable complexes with proteins, mechanisms similar to those reported for tannic acid and other polyphenols with esterified structures (Kurzbaum et al., 2019). PCA, with its phenolic hydroxyl groups, disrupts bacterial membranes and binds to DNA, impairing essential cellular functions (Li et al., 2022; Lou et al., 2012). Together, these mechanisms support MCC and PCA as effective antibacterial agents.
The anti-inflammatory activity of the EE extract, demonstrated by its inhibition of BSA denaturation, may involve interactions between polyphenols and proteins, a mechanism also described in previous studies (Kurzbaum et al., 2019; Rashedinia et al., 2023). Phenolic acids like PCA are known to stabilize protein structures by preventing denaturation and glycation, which are key processes in inflammation and oxidative stress (Rashedinia et al., 2023). Furthermore, PCA exhibits potent anti-inflammatory effects by reducing TNF-α expression (Rahman et al., 2021), which likely contributes to the anti-inflammatory activity of the EE extract and supports its potential to alleviate inflammation in infected skin.
In addition to these effects, the antioxidant activity of MCC and PCA is likely due to their phenolic hydroxyl (-OH) groups, which enhance electron donation ability and stabilize free radicals. This aligns with findings that such groups significantly contribute to the antioxidant potential of phenolic acids (Chen et al., 2020).
This study is the first to report the antimicrobial efficacy of MCC and PCA against C. acnes, highlighting their key role in the extract’s antibacterial activity. While other minor compounds may also contribute synergistically, further investigation is needed. Although our in vitro findings demonstrate the potential of E. pavieana extract, its efficacy in vivo may differ due to variations in skin microbiota and immune responses. A limitation of this study is its reliance on in vitro assays, which may not fully replicate human skin physiology. Additionally, while the extract showed low cytotoxicity in dermal fibroblasts, long-term toxicity studies and clinical trials are necessary to confirm its safety for topical use. Despite these limitations, the findings provide a strong foundation for developing E. pavieana-based skincare products.
5. CONCLUSION
This study highlights the potential of E. pavieana rhizome extract as a natural therapeutic agent for skin conditions characterized by microbial infection, oxidative stress, and inflammation. Given its low toxicity profile, the EE extract emerges as a safe and effective candidate for integration into skincare formulations. The high levels of bioactive phenolic compounds, particularly MCC and PCA, suggest significant therapeutic roles. These findings pave the way for developing innovative, natural-based skincare formulations that leverage the benefits of E. pavieana extract.
ACKNOWLEDGMENTS
The authors would like to thank Asst. Prof. Ekaruth Srisook of Burapha University for generously providing the tested standard (PCA and MCH) for the HPLC analysis, and the PI Lab members for their assistance in plant material preparation. They also extend their gratitude to the Research Unit of Natural Bioactive Compounds for Healthcare Products Development, and the Center of Excellence for Innovation in Chemistry (PERCH-CIC) under the Ministry of Higher Education, Science, Research, and Innovation, Thailand. Additionally, the authors appreciate the support from the Science Innovation Facility, Faculty of Science, Burapha University (SIF-IN-55300056, SIF-IN-63030345, SIF-IN-63030351, and SIF-IN-63030353).
AUTHORS’ CONTRIBUTIONS
Panata Iawsipo: Research concept and design, Collection and/or assembly of data, analysis and interpretation, Writing—critical revision and final approval. Tanikan Sangnim: Research concept and design, Writing and final approval of the article. Parinda Pratheep Na Thalang, Rachita Chernchom, Rattiya Bantao, and Kunlathida Luangpraditkun: Collection and/or assembly of data, analysis and interpretation, Final approval of the article.