1. Introduction
Cancer is defined by the uncontrolled growth of atypical cells that invade neighboring tissues or organs and spread to other regions of the body (Anonymous, 2025). Breast cancer is the most often diagnosed cancer (11.7%), accounting for 2.3 million new cases, followed by lung cancer (11.4%). Lung cancer is still the biggest cause of cancer-related mortality, accounting for around 1.8 million fatalities or 18% of all cancer deaths (Sung et al., 2021).
Tumor-derived cell lines are the predominant models utilized in cancer research, greatly advancing the comprehension of cancer biology in recent decades. Multiple studies have emphasized genetic disparities between cancer cell lines and tissue specimens (Ertel et al., 2006; Stein et al., 2004). A549 cells represent a commonly utilized human lung carcinoma epithelial cell line, initially isolated from lung tissue affected by adenocarcinoma, and are frequently employed in diverse fields of biomedical research (Domcke et al., 2013).
Hippocrates (Buqrat) is recognized for introducing the term “cancer.” It is derived from the Greek word ”cancrum,” which means crab. Hippocrates classifies nonulcer-forming tumors as carcinos and ulcer-forming tumors as carcinomas (Husain et al., 2018). Unani physicians characterize Sarat.ān (cancer) as a Sawdāwī warm (melancholic inflammation), arising from melancholic humors resulting from the combustion of Safrāwi madda (bile matter) (Sina, 2010). Cancer, also known as Waram-o-Salāba, is characterized by significant inflammation, lesions, and branches containing a melancholic humor (Madda-i-Sawdāwī) (Maseehi et al., 1995).
Cancer is characterised by significant inflammation that is firm and embedded in the tissue, along with tenderness and dryness.. The pressure exerted by the mass exacerbates the discomfort. At first, the growth may be diminutive, resembling a pea, but it has the potential to reach the size of a cantaloupe. The condition is deemed untreatable if it commences with intolerable agony. Nevertheless, the treatment may contribute to the regression of the growth’s progression if it occurs without causing significant pain (Hubal, 2005; Sina, 2010).
Contemporary cancer therapies encompass chemotherapy and radiation, both of which entail considerable side effects. Most chemotherapy agents target rapidly proliferating cells, impacting bone marrow, the gastrointestinal system, and hair follicles. These medications may induce myelosuppression, nausea, vomiting, gastrointestinal complications, diminished hematopoiesis, mucositis, alopecia, reproductive diseases such as sterility and infertility, as well as infusion reactions. Moreover, there is an increased susceptibility to infections because of impaired immune function (immunosuppression). (Amjad et al., 2024).
In the Unani medical tradition, various factors influence the onset and progression of cancer, particularly the increased synthesis of black bile (Sawdā) and its irregular transformations (Qamri, 2008; Razi, 1991). Galen states that cleansing the body of unhealthy humors requires the use of concoctive for black bile (Mundij-i-sawdā’) and purgative for black bile (Mushil-i-sawdā) (Razi, 1997).
In both in vitro and in vivo investigations, secondary metabolites from plants have exhibited anticancer properties. Phytochemicals such as Vinblastine, Vincristine, Camptothecin derivatives (Topotecan, Irinotecan), and Homoharringtonine are utilized in cancer therapy (Chandra et al., 2023). Currently, over 3,000 plant species globally have been documented for their anticancer properties. There is a necessity to develop therapeutically validated anticancer drugs from the traditional resources of herbs, minerals, and animals (Solowey et al., 2014). Natural antioxidants reduce the risk of developing conditions, that is, cancer, heart disease, and stroke (Prior et al., 2000). Plant secondary metabolites, including flavonoids and phenolics, exhibit significant free radical scavenging properties (Mathew et al., 2006).
A traditional polyherbal Unani formulation (PUF) containing 11 ingredients was evaluated for its efficacy and safety in a lung cancer cell line. The majority of the ingredients in the PUF have scientifically validated antioxidant and anticancer properties. This formulation is recommended in the management of melancholic diseases (Sawdāwī amrād) and serves as a concoctive for black bile (Mundij-i-sawdā), a purgative for black bile (Mushil-i-sawdā), and a purgative for phlegm (Mushil-i-balgham) (Table 1).
Table 1
Composition of the test formulation.
This historic Unani concoction, detailed in “Mundij wa mushil” by Majeed Ahmed Narvi, has been selected for examination to establish quality standards, authenticity, antioxidant activity, and anticancer potential by contemporary analytical methodologies (Narvi et al., 1983).
2. Materials and Methods
2.1. Crude drugs
The ingredients of the study drug were purchased from a licensed crude drug supplier in Hyderabad. The botanist at the Institute authenticated the crude drugs, and the voucher specimen nos. for Aftimoon, Bisfayej, Ustukhudus, Badranjboya, Berg-e-Gaozaban, Gul-e-Gaozaban, Badiyan, Asl-us-Soos, Maweez Munaqqa, Anjeer zard, and Gul-e-Surkh were assigned as SMPU/CRI-Hyd-15885, 15886, 15887, 15888, 15889, 15890, 15891, 15892, 15893, 15894, and 15895, respectively.
2.1.1. Preparation of the test drug
The PUF was manufactured in GMP-certified pharmacy licensed under 413/Unani of NRIUMSD, Hyderabad, as per the book titled Mundij-wa-Mushil. The individual ingredients were pulverized separately and then mixed to get the final composition. AQ, (1:1) HE, and ME extracts were prepared by mixing 285 g of powdered PUF with 100 mL of the corresponding solvent. The mixture was stirred at a speed of 50 rpm in a rotatory shaker incubator at a temperature of 37°C. Subsequently, the mixture underwent filtration and drying using a rotatory vacuum evaporator. The resulting product was then stored in a desiccator.
2.2. Organoleptic parameters and extractive values
Organoleptic parameters like texture, color, odor, and taste were evaluated, while extractive values of formulation in water, hydroethanol, and methanol were estimated (QSIMP, 2003; Singh et al., 2023; UPI, 2008).
2.3. Qualitative analysis of phytoconstituents of the extracts
The AQ, HE, and ME extracts of PUF were subjected to test the presence of therapeutically active chemicals like alkaloids, glycosides, fixed oil, etc., by using standard color reaction tests (Venkatesham et al., 2021).
2.4. Sample preparation
Samples of PUF powders (2 g each) were extracted with 20 mL of ethanol using an ultrasonicator for 30 min at a temperature of 45°C, performed in duplicate. The extracts were filtered utilizing Whatman’s No. 1 and 41 filter papers prior to being placed into vials for subsequent analysis (Akram et al., 2024).
2.4.1. Chromatographic conditions
The HPTLC chromatogram was executed on a 10 × 10 cm precoated silica gel 60 F254 HPTLC plate.
The samples were applied using 8 mm bands with the CAMAG Automatic TLC Sample Applicator IV, which is equipped with a 100 µl syringe. Twin trough glass chamber with dimensions of 20 cm by 10 cm.
Twin trough glass chamber (dimensions: 20 cm × 10 cm). The optimization of chamber saturation time for the mobile phase was determined to be 20 min at 25°C. The CAMAG TLC Scanner 4 was utilized to detect the densitogram in both absorbance and fluorescence modes at wavelengths of 254 nm, 366 nm, and 520 nm. The plate was applied for derivatization using a Vanillin–Sulfuric acid reagent, followed by scanning at a wavelength of 520 nm. The mobile phase employed for the chromatogram development consists of Toluene, Ethyl acetate, and Formic acid in a ratio of 7.5:2.5:0.01, and the resulting chromatogram was visualized utilizing the CAMAG TLC visualizer. The complete analysis was performed using WinCATS version 1.4.9.2001 operating software. Nitrogen served as the carrier gas for operation under high-pressure conditions. The lamps (Tungsten, Deuterium, and Mercury) were utilized for measuring absorbance values at 254 nm, 366 nm, and 520 nm (Akram et al., 2024).
2.5. Preparation of extract of PUF
The AQ, HE (1:1), and ME extracts of PUF, obtained through cold extraction and subsequently dried using a lyophilizer, were utilized in this study. A stock solution of 10 mg/mL was prepared by dissolving the desiccated extract in dimethyl sulfoxide (DMSO) (Kumar et al., 2023).
2.5.1. DPPH assay
An aliquot of 80 µl of 0.1 mM DPPH in methanol was combined with 20 µl of test extracts at concentrations between 3 and 500 μg/mL in a 96-well micro test plate. The reaction mixture was incubated in darkness, and absorbance was measured at 517 nm after a 30-min interval using a multimode reader (Infinite Pro M 200; Tecan, Austria). Ascorbic acid functioned as the positive control (Rumpf et al., 2023).
The calculation of free radical scavenging activity (% inhibition) was performed using the following formula:
2.5.2. ABTS scavenging activity
The ABTS test was conducted as previously outlined, with minor modifications. The formation of ABTS•+ was achieved by preparing a 7 mM solution of ABTS in distilled water, which was then oxidized using 2.45 mM potassium sulfate. The two stock solutions were combined in equal volumes, for 16 hours, to complete the reaction in dark room at normal temperature. One milliliter of ABTS•+ stock solution was prepared by diluting it with 60 mL of 100% methanol to create the working solution. Twenty microliters of test extracts at specified concentrations (15–500 μg/mL) were combined with 180 mL of ABTS•+ work reagent in a 96-micro well plate and incubated for 2 h at room temperature in the dark. Gallic acid was measured at 734 nm using a Tecan Multimode reader (Re et al., 1999).
The free radical scavenging activity (% inhibition) was calculated using the following formula:
2.6. Cytotoxicity Activity
A549 cells were cultured in a T-25 flask with a medium consisting of DMEM, 10% heat-inactivated FBS, 1% 200 mM L-glutamine, and 0.5% antibiotic anti-mycotic solution. The cells were cultured in a CO2 incubator with 95% air, 100% relative humidity, and 5% CO2 at 37°C. Cultures were subcultured every sixth day, and the media was refreshed three times weekly. Viable cell counts were assessed through the Trypan Blue exclusion method utilizing Hemacytometers (McCauley et al., 2013).
2.6.1. MTT Assay
The cytotoxicity was evaluated by the MTT assay, a colorimetric method. Cells were detached at approximately 90% confluency using 0.05% trypsin/EDTA. The cells were subsequently resuspended and inoculated into a 96-well plate at a volume of 200 μl per well. Equal cell quantities per well were subjected to varying doses of the test chemical on a logarithmic scale. Following 24-h incubation, the cells were rinsed with PBS, after which MTT (0.5 mg/mL in PBS) was added to each well and incubated at 37°C for 3 h. Formazan crystals were solubilized by adding DMSO (100 μL/well), and absorbance was measured at a wavelength of 570 nm using a Microplate Reader. The untreated cells served as the control group, exhibiting 100% viability. Paclitaxel was employed as the standard treatment. All concentrations were replicated in pairs, and results were derived from three measurements. The IC50 value will indicate the effectiveness of cell growth inhibition for each extract (McCauley et al., 2013).
The percentage of inhibition of the extract against the cell line was calculated using the formula provided below:
% Cell inhibition = 100 – % Cell viability
2.7. Quantitative analysis of total phenols (TPC)
The TPC was determined using the Folin–Ciocalteu reagent as described. Briefly, 30 μL of extracts were combined with 150 μL of 1:10 Folin–Ciocalteu reagent. After 5 mins of incubation, add 120 μL of 7% Na2CO3 and mix thoroughly. After 2 h of incubation in the dark, the absorbance at 750 nm was measured using a multimode reader (Infinite Pro M 200 Tecan, Austria). Gallic acid was utilized as the standard reference, and total phenols were reported in gallic acid equivalents (GAE) (Farasat et al., 2013).
2.8. Quantitative analysis of total flavonoid (TFC)
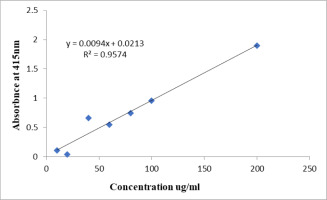
The amount of TFC was estimated using the quercetin standard curve and represented as quercetin equivalent (QE) using the aluminum chloride colorimetric test. To put it briefly, 10 μl of 10% aluminum chloride solution, 150 μl of 95% ethanol, and 10 μl of 1 M sodium acetate were added to a micro-well plate together with 50 μl of extracts (1000 µg/mL) and/or a standard solution of quercetin (50, 100, 150, 200, and 250 μg/mL) in 80% ethanol. After thoroughly mixing all the ingredients, they were incubated in the dark for 40 min. Using a multimode reader (Infinite pro M 200 Tecan, Austria), the absorbance was measured at 415 nm (Infinite pro M 200 Tecan, Austria) (Eddahhaoui et al., 2022).
3. Results
3.1. Organoleptic Evaluation and extractive values
Appearance: Coarse powder; Color: Light brown; Odor: Fragrance; and Taste: Slightly sweet. The mean concentrations of aqueous, hydroethanolic, and methanolic extracts (% w/w) were determined to be 4.78, 31.43, and 21.68, respectively.
3.3. Qualitative phytochemical analysis
The findings regarding the color reaction indicative of secondary metabolites are illustrated in Table 2. It has been noted that all extracts exhibited substantial levels of chemical constituents. The findings indicate the existence of therapeutically significant secondary metabolites, including alkaloids, flavonoids, saponins, tannins, proteins, and phenols, among others.
3.4. HPTLC of PUF
The HPTLC analysis demonstrated the existence of multiple phytochemical constituents in the ethanol extract of PUF, identified under two separate ultraviolet (UV) wavelengths. Under UV light at 254 nm, five significant major peaks were observed at Rf values of 0.01 (48.68%), 0.08 (8.15%), 0.18 (27.81%), 0.88 (8.23%), and 0.97 (7.13%). At 366 nm, seven major peaks were identified with Rf values of 0.01 (23.24%), 0.08 (62.88%), 0.13 (1.32%), 0.16 (1.04%), 0.21 (5.61%), 0.34 (4.16%), and 0.82 (1.16%). In addition, seven major peaks were observed with Rf values of 0.01 (24.28%), 0.14 (2.96%), 0.16 (6.53%), 0.35 (13.82%), 0.45 (7.84%), 0.65 (36.30%), and 0.98 (8.27%), as presented in Table 3. The chromatographic profiles demonstrate a unique chemical fingerprint of the ethanol extract of PUF, indicative of the solvent system used. The fingerprinting results, along with the 3D densitogram and the associated Rf values, as shown in Figure 1, validate the existence of active phytoconstituents in the extract. The extensive data gathered act as a valuable resource for identifying and characterizing phytoconstituents in PUF, offering a dependable reference for upcoming research (Christopher et al., 2024)
Figure 1
Rf values of HPTLC of alcoholic extract of polyherbal Unani formulation. (A) HPTLC finger print of PUF in alcohol extract at 254 nm, (B) 3D densitometry chromatogram of PUF at 254 nm, (C) peak table of PUF at 254 nm, (D) HPTLC finger print of PUF in alcohol extract at 366 nm, (E) 3D densitometry chromatogram of PUF at 366 nm, (F) peak table of PUF at 366 nm, (G) V-S reagent finger print of PUF in alcohol extract at 520 nm, (H) 3D densitometry chromatogram of PUF at 520 nm, and (I) peak table of PUF at 520 nm.

Table 3
Ethanolic extract of polyherbal Unani formulation observed phyto-constituents in various ultraviolet wavelengths.
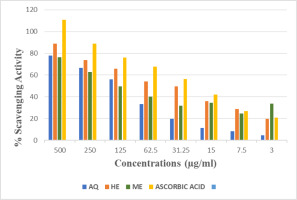
3.5. DPPH radical scavenging activity
All extracts of PUF showed dose-dependent antioxidant activity, increasing with higher concentration. Result showed highest activity in HE extracts with IC50 value 108 μg/mL, followed by ME and AQ extract when equated with ascorbic acid (Table 4 and Figure 2).
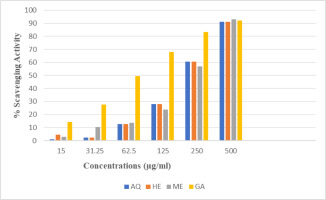
3.6. ABTS radical scavenging activity
All extracts of PUF showed dose-dependent antioxidant activity, increasing with higher concentration. Result showed almost similar activity in all extracts of the PUF (Table 5 and Figure 3).
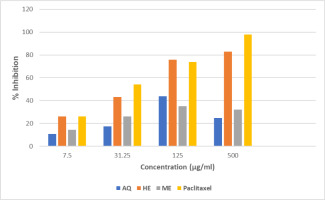
3.7. MTT assay
The cytotoxicity activity was evaluated at different concentrations extending 7.5, 31.25, 125, and 500 μg/mL, and subsequently IC50 was calculated. The highest activity was demonstrated by HE extracts with exhibited IC50 value of 100 μg/mL. The PUF has demonstrated the dose-dependent activity in all extracts (Table 6 and Figure 4).
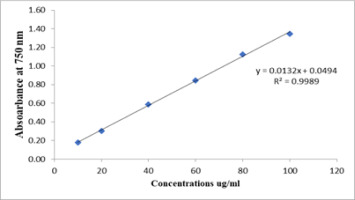
3.8. TPC
The TPC was measured using gallic acid (Figure 5) and expressed in milligrams of GAE. The extracts demonstrated a notable concentration of phenolic compounds, varying from 64.32±1.33 to 130.5±8.7 mg of GAE/g of extract. The methanolic extract exhibited the highest total phenolic concentration (Table 7).
3.9. TFC
Total TFC was estimated by Quercetin (Figure 6) and expressed as milligrams of QE. All the extracts contained a considerable amount of TFCs from 12±0.9 to 64±7.7 mg of QE/g of extract. Methanolic extract exhibited the highest total TFC (Table 7).
3.10. Pesticidal residue screening of PUF
The analysis of pesticide residues for PUF was conducted using the validated test method R-44 at Bureau Veritas India Testing Services, Private Limited, Sanath Nagar, Hyderabad, Telangana (Table 8). The testing was performed following the test method BVTS/FOOD/INS/SOP-040 for various pesticide residues, and the results are detailed in Table 8.
Table 8
Pesticide residue analysis for polyherbal Unani formulation.
4. Discussion
The main aim of this study was to investigate the anticancer and antioxidant properties of PUF in order to validate its claimed anticancer effects as outlined in Unani literature. In addition, we conducted phytochemical screening of extracts to identify the active compounds present in this formulation, alongside assessing certain physicochemical standardization parameters. In spite of the longstanding use of PUF by Unani physicians for the treatment of cancer, there is a dearth of scientific evidence to support these assertions. This research undertaken, represents an initial step towards validating the Unani tradition by investigating its anticancer properties using modern scientific approaches. Our expectation was that this initial investigation will set the stage for further clinical research into the potential therapeutic applications of this formulation.
Unani formulation “PUF” comprises 11 ingredients, out of that many possess anticancer and antioxidant properties as per Unani literature (Narvi et al., 1983). Recent studies showed that most of the ingredients have shown the anticancer and antioxidant properties (Table 9).
Table 9
Therapeutic action, uses, and anticancer and antioxidant activities of the ingredients of polyherbal Unani formulation.
| Drugs | Therapeutic actions and uses as per Unani | Antioxidant & anticancer activity studies |
|---|---|---|
| Cuscuta reflexa | Anti-inflammatory, tonic, concoctive of black bile and purgative of black bile. Cancer and melancholia (Ghani, 2011). | Anticancer and antioxidant activity (Elasbali et al., 2024; Singh et al., 2022). |
| Polypodium vulgare | Anticonvulsant, expectorant, and purgative of black bile and phlegm. Asthma and diseases of black bile and phlegm (Ghani, 2011). | Anticancer and antioxidant activity (Batur et al., 2020; Tabeshpour et al., 2023). |
| Lavandula stoechas | Tonic and purgative of black bile and phlegm. Schizophrenia and coryza (Ghani, 2011). | Anticancer and antioxidant activity (El Hachlafi et al., 2023; Syaj et al., 2025). |
| Melissa officinalis | Concoctive of black bile and sedative. Melancholic and phlegmatic disorders (Ghani, 2011). | Anticancer and antioxidant activity (Draginic et al., 2022; Kuo et al., 2021). |
| Borago officinalis | Purgative to black bile and tonic. Cough and pharyngitis (Ghani, 2011). | Anticancer and antioxidant activity (Michalak et al., 2023; Singh et al., 2017). |
| Foeniculum vulgare | Analgesic, concoctive of phlegm and black bile, and anti-inflammatory. Bronchial asthma and cough (Ghani, 2011). | Anticancer and antioxidant activity (DiNapoli et al., 2022; Ghasemian et al., 2020). |
| Glycyrrhiza glabra | Concoctive of phlegm, cleanses respiratory passage, concoctive of viscous humor. Bronchitis and diseases of phlegm and black bile (Ghani, 2011). | Anticancer and antioxidant activity (Caroline et al., 2023; Zhou et al., 2019). |
| Vitis vinifera | Tonic, concoctive of viscous humor, concoctive for phlegm and black bile. General debility, melancholic diseases, and cancer (Ghani, 2011). | Anticancer and antioxidant activity (Lantzouraki et al., 2020; Tsantila et al., 2024). |
| Ficus carica | Tonic, anti-inflammatory, concoctive, and demulcent for lungs. Schizophrenia (Ghani, 2011). | Anticancer and antioxidant activity (Ayoub et al., 2019; Purnamasari et al., 2019). |
| Rosa × damascena | Analgesic, purgative of phlegm and black bile, and anti-inflammatory. Syncope and pharyngitis (Ghani, 2011). | Anticancer and antioxidant activity (Shokrzadeh et al., 2017; Zahedi-Amiri et al., 2019). |
Growing evidence suggests that phytochemicals, derived from plants, possess therapeutic properties against various human diseases. Recent studies have shown that phytochemicals like flavonoids, phenols, coumarins, etc., exhibit promising efficacy in combating cancer with minimal toxicity. The proposed mechanism for their anticancer activity includes programmed cell death, cell migration, and senescence-like cell cycle arrest pathways. The desired effect may be achieved by modulating reactive oxygen species (ROS), the MAPK pathway, the DLC1 pathway, the NF-κB pathway, and glycolytic enzymes (Zheng et al., 2022). It was observed that PUF contains alkaloids, phenols, flavonoids, saponins, tannins, proteins, etc., in good proportion, which may be the reason for its antioxidant and cytotoxic properties. The findings of this investigation indicate that the HE extract exhibited the most significant free radical scavenging activity among the three extracts in DPPH as well as ABTS assay.
The research revealed that various extracts of PUF exhibited differing degrees of cytotoxicity on A549 cells, with the HE extract showing the highest level. All extracts of PUF had cytotoxic effects that were dependent on concentration. The maximum TPC was seen in the HE extract, but the highest TFC was recorded in the ME extract of PUF. The substantial concentration of TPC and TFC may corroborate the antioxidant and anticancer efficacy of the PUF. The findings of this investigation indicate that PUF exhibited a significant cytotoxic impact comparable to the control medication paclitaxel. This strongly indicates its potential efficacy on the A549 cell line.
5. Conclusion
The PUF contains good amount of TPC and TFC along with alkaloids, saponins, tannins, proteins, etc. The presence of secondary metabolites justifies the antioxidant and cytotoxic effects of PUF against the A549 cell line. The best free radical scavenging and anticancer activity was demonstrated by hydroethanolic extracts of PUF. Emphasizing the benefits of the Unani system, particularly its reliance on natural and easily metabolized substances, could improve comprehension and possibly foster greater acceptance of traditional medicinal practices. The findings reinforce the Unani methodology of employing natural drugs in their unaltered state. Because of the preliminary nature of the study, additional research, particularly involving animal models and subsequent clinical trials, is recommended.