1. Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune condition that gradually damages joints, tendons, and ligaments of the body (Lee and Weinblatt, 2001). Alongside osteoarthritis (OA), RA is recognized as a progressive disorder marked by persistent joint pain, inflammation, and a decline in physical function. Clinical features of RA include widespread joint involvement, morning stiffness, and degeneration of both cartilage and bone, often leading to deformity and disability. The global prevalence of RA is estimated between 0.5 and 1.0% (Smolen et al., 2018). In spite of advances, joint pain associated with OA and RA remains inadequately controlled, especially among older individuals among whom prevalence is rising (Martel-Pelletier et al., 2016). Characteristic symptoms include inflammation of the synovial lining, restricted movement, and chronic pain. The nature of arthritis-related pain is complex, involving multiple pain
mechanisms including nociceptive and neuropathic elements. This complexity, coupled with disease burden, makes RA a significant contributor to disability and global healthcare costs. RA is driven by complex immune mechanisms, with inflammation and progressive destruction of tissues being hallmark features, though the exact cause remains elusive. The disease is marked by elevated levels of cytokines including IL-6, IL-1â, and TNF-á, which disrupt cartilage integrity and promote joint damage through oxidative stress and inflammatory mediator release. These proinflammatory factors, along with NF-êB, are central to the chronic progression of RA (Cui et al., 2020). IL-6 contributes to inflammation and new blood vessel formation, making it a potential therapeutic target. TNF-á amplifies joint inflammation by enhancing immune cell recruitment and promoting expression of adhesion molecules within synovial tissues. The result is joint degradation via transcriptional activation of destructive cytokines and signalling proteins. Oxidative stress further worsens RA and OA by generating reactive oxygen species (ROS), which damage joint tissues and enhance inflammatory responses (García-González et al., 2015).
The CFA-induced arthritis model is commonly used to simulate human RA in rats, as it elicits immune responses involving T-cells and macrophages, resulting in chronic joint inflammation. This model mirrors key aspects of RA, including elevated levels of inflammatory mediators such as NF-êB, IL-1â, IL-6, and TNF-á, which contribute to cartilage and joint destruction. However, many preclinical models lack consistent clinical relevance, highlighting the need for more reliable systems (Mogil and Crager, 2004). NSAIDs and biologics are widely used in managing RA, yet their limitations are well-documented. These include suboptimal control of disease progression, adverse side effects, and high treatment costs, especially with biologics. A significant proportion of patients fail to respond adequately to TNF-á blockers or experience toxicity with prolonged use of NSAIDs (Harirforoosh et al., 2013). Because of these challenges, there is growing interest in natural therapies. Plant-based agents possessing antioxidant and anti-inflammatory properties offer a promising alternative for reducing oxidative damage and inflammation in arthritic conditions. Traditional and herbal remedies are increasingly explored for their efficacy and reduced side effects (Tong et al., 2014). Nevertheless, progress in developing more effective pain treatments has been limited, reinforcing the need for safer and more effective strategies for managing persistent RA pain (Mogil et al., 2010).
This study focuses on examining the therapeutic impact of Scitican-50 tablet in rat models of arthritis triggered by monosodium iodoacetate (MIA) and Complete Freund’s Adjuvant (CFA). Contemporary treatment strategies increasingly focus on poly-pharmacology, where multiple molecular targets are modulated simultaneously. Plant-based extracts are promising in this context because of their complex chemical composition and capacity to affect various biological pathways.
Existing treatments for rheumatoid and OA have limitations like side effects, high cost, and incomplete pain relief, especially in low-resource settings. Polyherbal formulations like Scitican-50 show promise but lack sufficient preclinical safety and efficacy data. This study aims to fill this gap by evaluating Scitican-50’s analgesic, anti-inflammatory, and chondroprotective effects in MIA and CFA-induced arthritis models in rats. These findings will help assess its potential as a multi-targeted plant-based alternative for the management of joint pain.
Scitican-50, developed by Sudhatatva Pharmacy, Dr. D. Y. Patil College of Ayurved and Research, Pune, is a polyherbal formulation containing Vijaya, Shu. Hingul, Marich, Shu. Kuchala, and Sunthi. These herbs, known for treating anxiety and other ailments in traditional medicine, lack preclinical evaluation for safety and efficacy. This study aims to assess its toxicity and joint pain–relieving activity effects. Scitican-50 (500 mg) is composed of standardized extracts of Vijaya (Cannabis sativa, 50 mg), Shuddha Hingul (Ferula asafoetida, 50 mg), Marich (Piper nigrum, 50 mg), Shuddha Kuchala (Strychnos nux-vomica, 50 mg), and Sunthi (Zingiber officinale (ZO) 50 mg). The formulation is blended with suitable pharmaceutical excipients to make up the total weight of 500 mg per tablet.
Several plant-based compounds have demonstrated notable anti-inflammatory and analgesic properties. Ferula asafoetida resin exhibits diverse therapeutic effects, including antioxidant and antispasmodic actions (Mahendra and Bisht, 2012; Nabavi et al., 2011). Piper nigrum (black pepper), rich in piperine, has shown significant anti-inflammatory activity and pain-relieving potential, particularly through novel nanoparticle formulations (Damanhouri, 2014; Doucette et al., 2013; Mani et al., 2016). Cannabis Sativa–derived cannabidiol (CBD) effectively reduced osteoarthritic pain and improved mobility in dogs, with liposomal formulations enhancing its bioavailability (Verrico et al., 2020). Similarly, raw and Kanji-processed Kupeelu (Strychnos nux-vomica) selectively alleviated chronic inflammatory pain in formaldehyde-induced models (Mitra et al., 2011), while ZO may exert effects via inhibition of COX and LOX pathways (Grzanna et al., 2005).
This study aims to explore the effectiveness and safety of Scitican-50, a polyherbal tablet, by investigating its impact on pain relief, side effect profile, and mechanisms of action. If proven effective, this approach could offer a safer, more holistic treatment option for individuals suffering from joint pain, reducing the burden of adverse effects associated with conventional treatments.
2. Materials and Methods
Standard Drugs and Kits: Diazepam [Batch No. DP-2298; Expiry: June 2025] was procured from Aagya Biotech Pvt. Ltd. ELISA kits for the estimation of pro-inflammatory cytokines, including TNF-á (Catalog No. KB3145) and IL-1â (Catalog No. KLR0119), were obtained from Krishgen BioSystems. In addition, Scitican-50 tablets were sourced from Sudhatatva Pharmacy, Pune. The chemicals used in the study were MIA purchased from Sigma Aldrich (or relevant company) and used at 1.5 mg/50 µl for intraarticular injection; CFA containing heat-killed mycobacterium tuberculosis was used for arthritis induction; Bradford reagent was procured from Sigma Aldrich; bovine serum albumin and Ellman’s reagent were obtained from Himedia, Mumbai; and formaldehyde was purchased from Research Lab, Mumbai. Various laboratory glassware and tools were utilized, including homogenizing vessels, volumetric flasks, mortar and pestle, test tubes, micropipettes, measuring cylinders, beakers, and microplates.
The instruments employed during the experimental procedures included a Vernier caliper (Zhart, India), an analgesiometer (Eddy’s Hot Plate) from Inco Ambala, a microplate reader (Biotech Epoch, Fisher Scientific, USA), an analytical weighing balance (Shimadzu AX-200), a centrifuge (Remi Scientific, Mumbai), and a UV-Visible spectrophotometer (Shimadzu-1800, Japan).
The experimental protocol was reviewed and approved by the Institutional Animal Ethics Committee (IAEC) of Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune, under Protocol No. DYPIPSR/IAEC/Nov/23-24/P-17. All experimental procedures were conducted in accordance with the Committee for Control and Supervision of Experiments on Animals (CCSEA) guidelines, Government of India, 2022. The animals were housed in a registered animal facility (Registration No. 198/PO/Re/S/2000/CCSEA) at the same institution. All investigators followed ethical standards and adhered to the principles of replacement, reduction, and refinement (3Rs) to ensure the humane and responsible use of laboratory animals. The sample size of eight rats per group was determined based on consistency with previous published studies (Chen et al., 2020) using CFA- and MIA-induced arthritis models, where group sizes of five to eight animals provided sufficient statistical power to detect treatment effects on pain behavior, paw edema, and inflammatory biomarkers. Although a formal a priori power analysis was not performed, this sample size is widely accepted in exploratory preclinical studies and was approved by the IAEC.
The CFA and MIA models were chosen for their ability to mimic key features of human RA and OA, respectively. CFA induces immune-mediated joint inflammation with synovitis and systemic activation (Guingamp et al., 1997), while MIA causes chondrocyte toxicity and cartilage degradation, replicating osteoarthritic pain (Orita et al., 2011). Both models have limitations: the response of the CFA is acute and may not fully mimic chronic autoimmunity, and MIA-induced damage is rapid and chemical, not mechanical. In spite of these drawbacks, they remain widely used for testing analgesic and disease-modifying treatments.
2.1. Acute oral toxicity study
An acute oral toxicity study was conducted following OECD guideline 423 to assess the safety of the test formulation. Female Wistar rats were divided into two groups: a normal control group (n=3) receiving the vehicle (1 mL, p.o.) and a test group (n=6) administered the formulation at a limit dose of 2000 mg/kg orally. Post-administration, animals were observed individually, focusing on the first 30 minutes to 4 hours, followed by periodic checks for 24 hours and daily for 14 days. On Day 15, a gross necropsy was performed to examine vital organs for pathological changes. Monitoring was conducted twice daily during the peak arthritis induction phase (first 7 days) and daily thereafter. Two experimental models were used to induce joint pain like the MIA model, involving intra-articular injection to induce OA-like symptoms, and the CFA model, using subcutaneous injection to induce inflammatory arthritis (Tables 1 and 2).
Table 1
Animal grouping for the monosodium iodoacetate model.
Table 2
Animal grouping for the CFA model.
2.2. Experimental animals and housing conditions
Healthy female Wistar rats (aged 6–8 weeks, weighing 180–200 g) were procured from Crystal Biological Solutions, Pune. Animals were housed in groups of four in opaque polypropylene cages (28 × 21 × 14 cm) with autoclaved, dust-free, paddy husk as bedding. The animals were maintained under standard laboratory conditions, with a 12-hour light/dark cycle, ambient temperature of 22 ± 5 °C, and relative humidity ranging between 50 and 70%. Rats had free access to a standard pellet diet and water ad libitum.
2.3. Induction of arthritis and drug treatment
Arthritis was induced using two preclinical models: the CFA model for inflammatory arthritis and the MIA model for OA. Animals were handled gently and acclimatized prior to experimentation to reduce stress. To minimize pain and discomfort, the dose of CFA and MIA used was the minimum required to induce arthritis symptoms consistent with the study objectives. In the CFA model, a single intra-plantar injection of 150 µL CFA was administered into the right hind paw. Animals were divided into six groups (n=8). The normal control group received 0.25% sodium carboxymethyl cellulose (Na-CMC, 10 mL/kg, p.o.), and the disease control group received CFA only. The standard group was treated with diclofenac sodium (10 mg/kg, p.o.). Treatment groups received Scitican-50 tablets orally at doses of 50, 100, and 200 mg/kg.
In the MIA model, OA was induced by a single intra-articular injection of MIA (1.5 mg/50 µL) into the knee joint. Groups and treatment protocols mirrored those in the CFA model, with Na-CMC, MIA-only, diclofenac, and three Scitican-50 dose groups (50, 100, 200 mg/kg, p.o.).
2.4. Evaluation parameters
Assessment of therapeutic efficacy included behavioral, biochemical, and histological parameters. Food and water intake, along with body weight, were recorded pre-induction and twice weekly until Day 28. Pain threshold was measured on Days 0, 7, 14, 21, and 28 using the hot plate method. Knee joint thickness and paw edema were evaluated daily using a digital vernier caliper and visual inspection, respectively. On completion of the study, hematological parameters (CBC) were assessed, and serum samples were analyzed for pro-inflammatory cytokines TNF-á and IL-1â using ELISA. Radiographic examination of knee joints was performed, followed by histopathological analysis to evaluate cartilage integrity, synovial inflammation, and bone structure.
2.5. Data analysis
All data were expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism version 8.0 (GraphPad Software Inc., San Diego, CA, USA). Comparisons between multiple groups were conducted using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test to determine statistical significance. A p-value of <0.05 was considered statistically significant. The level of significance is indicated as follows: p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****), ns: not significant. No animals, experimental units, or data points were excluded from any of the analyses.
3. Results
3.1. Pain threshold—Eddy’s hot plate method
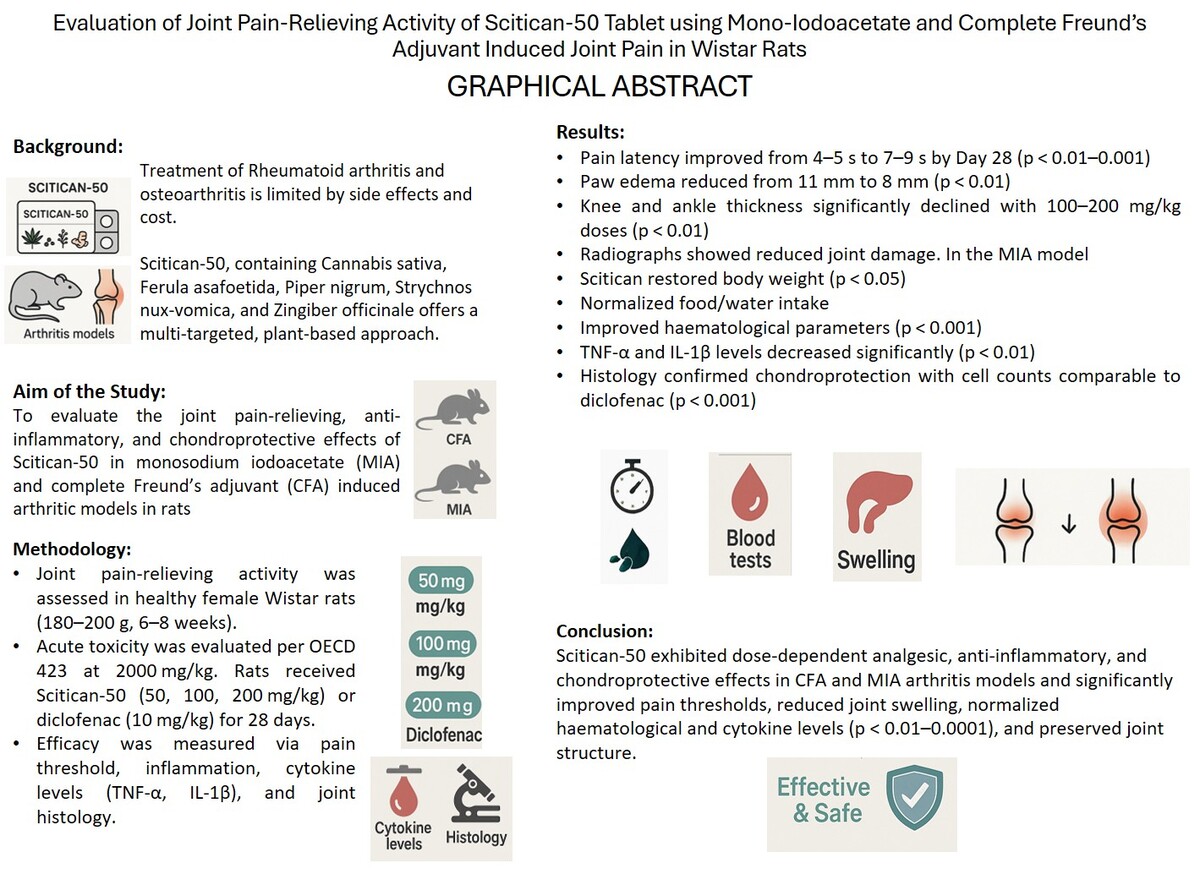
Following induction of arthritis, latency of pain significantly decreased from 7–8 to 4–5 seconds (p < 0.0001), indicating increased pain sensitivity. Diclofenac restored latency to normal by Day 14. Scitican-50 showed moderate improvement (p < 0.01) by Days 14 and 21, reaching 7 seconds by Day 28, while Scitican-100 and -200 restored latency to 9 seconds by Days 21–28, comparable to diclofenac. These results highlight Scitican’s dose-dependent analgesic efficacy in arthritic rats (Figure 1A).
3.2. Paw edema
Following the induction of CFA, paw edema significantly increased to 11 mm by Day 7 and persisted through Day 28 (p < 0.0001). Diclofenac effectively reduced edema to 7.5 mm by Day 21. Scitican-50 showed moderate reduction to 9 mm, while Scitican-100 and -200 significantly decreased edema to 7.8 and 8 mm, respectively (p < 0.01) by Day 21. However, by Day 28, reductions plateaued and were not statistically significant, indicating partial recovery or limited effect (Figure 1B).
3.3. Knee joint thickness
Induction of arthritis significantly increased the thickness of knee joint to 14 mm by Day 7 (p < 0.0001). Diclofenac reduced swelling to near-normal levels (10.2 mm) by Day 21. Scitican-50 moderately decreased thickness to 11.5 mm (p < 0.01) by Day 21 and maintained ~12 mm by Day 28 (p < 0.05). Scitican-100 and -200 effectively attenuated inflammation, reducing thickness to ~11.2 mm by Day 28, demonstrating dose-dependent anti-inflammatory effects (Figure 1C).
3.4. Ankle thickness
The thickness of the ankle significantly increased to 7.8 mm in the disease group from Day 14 onward (p < 0.001), indicating severe inflammation. Diclofenac reduced swelling to 5.2 mm by Day 21. Scitican-50 and –200 lowered the thickness of the ankle to ~5.4–5.5 mm by Day 28, while Scitican-100 showed significant reduction by Day 21 (p < 0.01), comparable to diclofenac’s effect (Figure 1D).
3.5. Radiographic evaluation of knee joints
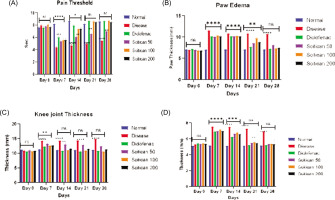
Radiographs showed intact joints in the normal group, while the disease control group exhibited severe pathology with marked swelling of soft tissues and periosteal reaction (score 3), plus narrowing of joint space and erosion of bone (score 2). Diclofenac significantly improved joint condition with mild swelling and reduced bone damage (score 1). Scitican-50 demonstrated dose-dependent radiological improvements: 50 mg/kg showed moderate pathology, 100 mg/kg reduced periosteal reaction and erosion, and 200 mg/kg provided substantial protection comparable to diclofenac. These findings highlight Scitican’s disease-modifying potential in inflammatory arthritis (Table 3, Figure 2).
3.6. MIA-induced joint pain Model
3.6.1. Effect of scitican-50 tablet on the animal’s body weight
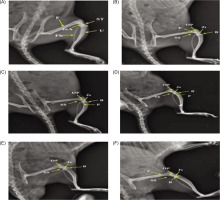
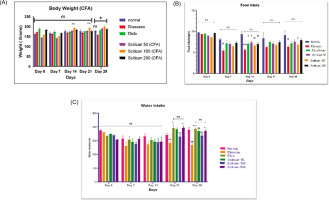
In the MIA model, disease rats showed weight loss from 167 g to 160 g by Day 28, with Scitican-50 at 50 mg and 200mg dose significantly improving body weight (188–195 g; p<0.05). Food intake declined significantly in disease controls on Day 14 (p < 0.01) but improved with Scitican-50 at 100 mg and 200 mg treatments (p < 0.05). Water intake decreased significantly on Day 21 and Day 28 (p < 0.01) in sick rats, while Scitican-50 at 50 mg and 100 mg dose restored intake by Day 21 (p < 0.05–0.01), supporting recovery and hydration (Figure 3).
3.6.2. Hematological findings in MIA-induced joint pain model
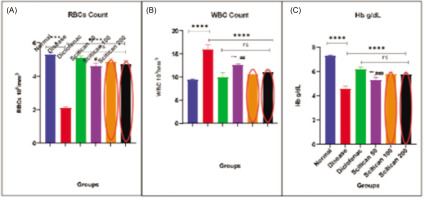
In the MIA-induced arthritis model, significant hematological alterations were observed. The disease control group showed elevated RBC (5 million/μL) and WBC (16/μL) counts and decreased Hb (4.6 g/dL) versus normal controls (RBC: 2 million/μL, WBC: 9/μL, Hb: 7.2 g/dL), indicating systemic inflammation and anemia (p < 0.001 for RBC, p < 0.0001 for WBC and Hb). Scitican-50 treatment demonstrated dose-dependent normalization of these parameters. Scitican-100 and -200 mg/kg notably reduced WBC counts (11–11.2/μL), lowered RBCs (4.6–4.7 million/μL), and improved Hb (5.8 g/dL), with effects comparable to diclofenac (RBC p < 0.001, WBC and Hb p < 0.0001). These findings support Scitican’s anti-inflammatory and hematoprotective potential in arthritic condition (Figure 4A–C).
3.6.3. MIA-induced joint pain model—cytokine analysis
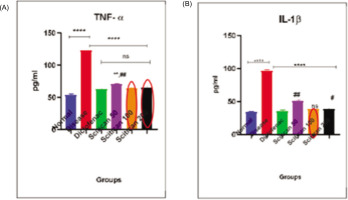
In the MIA-induced arthritis model, TNF-á and IL-1â levels were significantly elevated in disease controls (TNF-á: 120 pg/mL, IL-1â: 100 pg/mL, p < 0.0001) versus normal. Scitican-50 treatment dose-dependently reduced these cytokines, with Scitican-50 lowering TNF-á to 68 pg/mL and IL-1â to 66 pg/mL (p < 0.01), while Scitican-100/-200 achieved reductions comparable to diclofenac (TNF-á ~62–64 pg/mL, IL-1â ~64 pg/mL) (Figure 5A and 5B).
3.6.4. Histopathological evaluation of ankle joints in MIA-induced OA model
Histopathology of MIA-induced OA showed intact joint architecture and high chondrocyte counts in normal controls (2595 cells). Disease controls exhibited severe degeneration and reduced cell viability (973 cells). Diclofenac preserved joint integrity (2423 cells). Scitican-50 demonstrated dose-dependent chondroprotection, with 100 mg/kg maintaining near-normal structure and cell count (2144), comparable to diclofenac. The 200 mg/kg dose showed moderate protection (1809 cells), while 50 mg/kg offered partial recovery (1041 cells). These results underscore Scitican’s effective cartilage protection, optimally at 100 mg/kg (Figure 6).
3.7. CFA-Induced joint pain model
3.7.1. Effect of scitican-50 tablet on the animal’s body weight
In the CFA model, disease controls showed decreased body weight (170 to 158 g by Day 28) and reduced food and water intake (p < 0.001 for food on Day 14). Scitican-100 significantly improved body weight by Days 14 and 21 (187 g; p < 0.01) and increased food intake (p < 0.05 on Day 14). Water intake also improved significantly in diclofenac, Scitican-50, and Scitican-100 groups by Day 28 (p < 0.05), indicating alleviation of joint pain and enhanced health (Figure 7).
3.7.2. Hematological findings in CFA-induced joint pain model
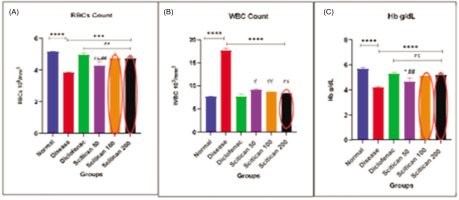
In the CFA model, disease rats showed significant anemia with reduced RBCs (3.8 vs. 5.2 million/μL, p < 0.001) and elevated WBCs (17.5 vs. 7.4 cells/μL, p < 0.0001), indicating chronic inflammation. Scitican-100 mg and –200 mg doses effectively restored RBCs (4.8 million/μL) and lowered WBCs (~8.7–8.8 cells/μL), comparable to diclofenac (RBC: 5.0; WBC: 7.5). Hb was paradoxically higher in disease controls (5.6 vs. 4.2 g/dL, p < 0.0001), with Scitican-50 and diclofenac showing modest normalization. These results affirm Scitican’s anti-inflammatory and hematoprotective effects (Figure 8).
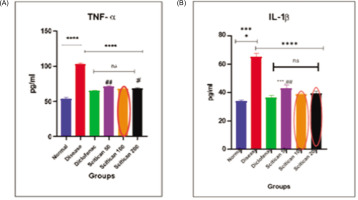
3.7.3. CFA-induced joint pain model—cytokine analysis
In the CFA model, TNF-á and IL-1â levels were significantly elevated in disease controls (TNF-á: 90 pg/mL, IL-1â: 65 pg/mL, p < 0.0001) versus normal. Scitican-50 treatment dose-dependently reduced TNF-á to 50–40 pg/mL (p < 0.01) and IL-1â to 42–36 pg/mL (p < 0.05), approaching diclofenac’s potent suppression (TNF-á: 30 pg/mL, IL-1â: 34 pg/mL). These results confirm Scitican’s anti-inflammatory efficacy in chronic arthritis (Figure 9).
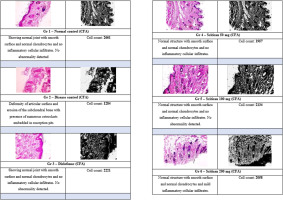
3.7.4. Histopathological evaluation of ankle joints in CFA-induced arthritis model
Histology revealed intact joint structure and high chondrocyte counts in normal controls (2661 cells). CFA-induced arthritis caused severe joint damage and reduced cell viability (1284 cells). Diclofenac restored normal morphology with increased cell count (2221). Scitican-50 showed dose-dependent chondroprotection: 100 mg/kg preserved joint architecture and inflammation absence (2134 cells), closely matching diclofenac. The 200 mg/kg dose of Scitican-50 maintained integrity with mild inflammation (2058 cells), while 50 mg/kg offered moderate protection (1957 cells). These findings support Scitican’s anti-inflammatory and chondroprotective potential (Figure 10).
Several herbal formulations have shown anti-inflammatory and analgesic effects in arthritis models, mainly because of bioactive phytochemicals with antioxidant and immunomodulatory properties. However, most lack thorough evaluation regarding standardization, dose-dependent efficacy, and safety. Scitican-50, a polyherbal tablet combining Cannabis sativa, Ferula asafoetida, Piper nigrum, Strychnos nux-vomica, and ZO, is proposed to provide superior therapeutic outcomes through multitargeted action. Our findings suggest that Scitican-50 significantly reduces pain and inflammation while preserving joint integrity. Acute toxicity testing at 2000 mg/kg showed no observable adverse effects. However, further safety evaluation at therapeutic doses remains essential. These outcomes highlight its potential as a safer and more effective alternative to existing herbal treatments for joint pain.
4. DISCUSSION
Our study demonstrated that Scitican-50 tablets exert dose-dependent analgesic, anti-inflammatory, hematoprotective, and chondroprotective effects in both CFA- and MIA-induced arthritis models. Scitican-50 significantly improved pain thresholds, reduced paw edema, normalized hematological parameters, lowered pro-inflammatory cytokines (TNF-á, IL-1â), and preserved joint integrity, showing efficacy comparable to diclofenac. Although the precise mechanism of Scitican-50 is not fully elucidated in this study, its dose-dependent suppression of TNF-á and IL-1â suggests immunomodulatory action. Based on its herbal composition, it may exert effects through inhibition of NF-êB and MAPK signaling pathways, suppression of oxidative stress, and modulation of nociceptive neurotransmission mechanisms previously associated with several phytoconstituents present in anti-inflammatory formulations.
Compared to Jobelyn®, which reduced CFA-induced paw edema, oxidative stress, and neurobehavioral impairments by lowering TNF-á and IL-6, Scitican-50 similarly suppressed pro-inflammatory cytokines and reversed joint inflammation with additional normalization of hematology and joint histology (Omorogbe et al., 2018). In relation to D-carvone, which mainly acted through antioxidant and anti-inflammatory pathways in CFA arthritis, Scitican-50 showed broader efficacy across CFA and MIA models, including dose-dependent pain relief and structural preservation comparable to diclofenac (Chen et al., 2020). Scitican-50 also paralleled the combination of Shinbaro and celecoxib, which alleviated CFA pain via central spinal modulation and immune suppression. However, Scitican-50 demonstrated robust peripheral effects by reducing joint inflammation, restoring systemic health indicators, and preserving joint architecture without requiring combination therapy (Jang et al., 2025). Unlike pregabalin, which was evaluated primarily by dynamic gait analysis, Scitican’s multidimensional assessment included pain latency, edema, cytokines, hematology, and histopathology, confirming consistent dose-dependent benefits in both arthritis models (Ängeby Möller et al., 2018). While botulinum toxin A (BoNT-A) showed localized molecular effects after a single intra-articular injection, Scitican-50 provided systemic, dose-dependent disease modification with chondroprotective and hematological improvements over 28 days (Yoo et al., 2014). Similarly, curcumin-loaded nanoparticles reduced inflammation and preserved joints in CFA arthritis, but Scitican’s dual model evaluation and hematological focus provide a broader therapeutic profile (Arora et al., 2015).
Paullinia pinnata extracts reduced inflammation and pain by inhibiting cytokines and oxidative stress, whereas Scitican-50 additionally demonstrated systemic recovery and joint structural protection (Tseuguem et al., 2019). Finally, in comparison to CN treatment, which lowered TNF-á, IL-6, and VEGF in arthritis models, Scitican-50 showed detailed dose-dependent improvements in cytokines and joint histology, suggesting complementary mechanisms of action (Pan et al., 2017). Overall, Scitican’s comprehensive anti-arthritic efficacy across multiple parameters supports its potential as a disease-modifying agent, combining analgesic, anti-inflammatory, hematological, and chondroprotective properties comparable to or exceeding existing treatments. Our study on Scitican-50 demonstrated significant dose-dependent analgesic, anti-inflammatory, hematological, and chondroprotective effects in MIA and CFA-induced arthritis models, comparable to the standard drug diclofenac. These findings align with various external studies investigating natural and synthetic anti-arthritic agents, though notable differences exist.
Similar to Scitican, Nitazoxanide (NTZ) improved body weight, reduced paw swelling, and normalized hematological parameters in CFA rats, also demonstrating inhibition of STAT-3 and NF-êB pathways—mechanisms not explicitly examined in our study but likely involved given the cytokine reductions observed (Prajapati and Doshi, 2025). Astragali radix extract showed marked pain reduction and joint protection, with repeated dosing preventing hypersensitivity; Scitican-50 exhibited comparable chondroprotection and cytokine suppression but did not assess hypersensitivity prevention (Maresca et al., 2017). Eriobotrya japonica leaf extract (EJLE) reduced paw edema and inflammation through specific triterpenoids affecting leukocyte migration and nitric oxide production. While Scitican’s effects were demonstrated primarily via dose-response in pain and inflammation parameters, EJLE provides a clearer phytochemical mechanism (Kuraoka-Oliveira et al., 2020). Moringa oleifera extracts showed synergistic analgesic effects, similar to Scitican’s dose-dependent pain relief, though the synergistic and prophylactic benefits of Moringa were not explored in our work (Manaheji et al., 2011).
In contrast, Ferula asafoetida extract exhibited minimal therapeutic effects with mild organ toxicity, highlighting Scitican’s favorable safety and efficacy profile (Goudah et al., 2015). Silver nanoparticles stabilized with Piper nigrum (S-AgNPs) showed potent anti-arthritic activity, suppressing NF-êB and TNF-á, paralleling Scitican’s cytokine modulation though with a distinct nano-based delivery mechanism (Mani et al., 2016). Similarly, Piper nigrum extracts demonstrated rapid analgesic and anti-inflammatory effects, while Scitican’s effects developed progressively over treatment duration (Tasleem et al., 2014). Ginger combinations matched diclofenac in pain relief but with superior gastric mucosal protection, a safety aspect not addressed in our Scitican-50 study (Drozdov et al., 2012). Juniperus sabina extract reduced inflammation and cytokines, supporting traditional herbal use for arthritis much like Scitican’s dose-dependent efficacy (Zhao et al., 2016). Peperomia pellucida extract showed anti-inflammatory and antioxidant effects alongside joint protection; while Scitican-50 also reduced inflammation and joint damage, its antioxidant capacity requires further evaluation (Uwaya et al., 2024).
Overall, Scitican’s multifaceted benefits across pain, inflammation, hematology, and histopathology corroborate findings from diverse natural and synthetic agents, highlighting its promise as a disease-modifying therapy for inflammatory arthritis. However, while acute toxicity testing at a high dose (2000 mg/kg) showed no adverse effects, the long-term safety and potential risks associated with chronic administration of herbal constituents remain unclear. Detailed toxicological profiling, particularly at therapeutic doses over extended periods, is necessary to confirm the formulation’s safety for clinical use. This study supports advancing Scitican-50 to clinical trials for the treatment of inflammatory arthritis. Future research should clarify its molecular mechanisms, focusing on NF-êB and MAPK pathways. Long-term safety and herb-drug interaction studies are also needed.
5. CONCLUSION
This study demonstrates that Scitican-50 tablets exert significant dose-dependent analgesic, anti-inflammatory, hematoprotective, and chondroprotective effects in both MIA- and CFA-induced joint pain models in Wistar rats. Scitican-50 improved pain thresholds, reduced edema, normalized hematological parameters, and suppressed key pro-inflammatory cytokines (TNF-á, IL-1â), comparable to diclofenac. Radiographic and histopathological analyses confirmed its ability to preserve joint and cartilage integrity, indicating disease-modifying potential. In addition, Scitican-50 enhanced body weight and food and water intake, reflecting systemic health benefits. Among the doses tested, 100 mg/kg showed the most balanced and effective outcomes across all parameters, providing optimal pain relief and joint protection without adverse effects. Thus, 100 mg/kg is identified as the major effective dose of Scitican-50 for managing inflammatory and osteoarthritic joint pain.
5.1. Study limitations
This study provides strong preliminary evidence of Scitican-50’s promising dose-dependent effects on pain relief, inflammation reduction, and joint protection in arthritis models. However, while CFA and MIA models are widely accepted, they do not fully replicate the complexity of human RA or OA, which may limit direct translation of findings. Despite these limitations, the comprehensive multiparameter evaluation and consistent dose–response effects highlight the therapeutic potential of Scitican-50, encouraging further mechanistic and clinical studies to elucidate the molecular targets of Scitican-50, long-term safety and toxicity evaluations, and clinical trials to confirm efficacy and safety in human subjects. These steps will help bridge the gap between preclinical findings and clinical application.