1. INTRODUCTION
The Tenasserim Range in Western Thailand, part of the Indo-Malayan mountain system, is a biodiversity hotspot, particularly noted for its abundance of medicinal plants, including species from the Zingiberaceae family. Commonly referred to as the ginger family, this group encompasses herbaceous plants with aromatic rhizomes and essential oils. These plants thrive in tropical environments, and Thailand’s favorable climatic conditions support over 300 species across 26 genera.
The Zingiberaceae family is renowned for its medicinal and aromatic properties, contributing significantly to traditional medicine and cultural practices across Southeast Asia. Species such as Boesenbergia rotunda (fingerroot) and Curcuma longa (turmeric) are integral to Thai cuisine and traditional medicine. These plants are commonly used to treat various ailments, including rheumatism, muscle pain, fever, gout, gastrointestinal disorders, flatulence, indigestion, stomachache, and osteoporosis (Eng-Chong et al., 2012; Gu et al., 2017; Saah et al., 2021). Their bioactive compounds exhibit diverse therapeutic properties such as antimicrobial, antimalarial, antioxidant, anti-tyrosinase, anti-inflammatory, antitumor, anticancer, anti-diabetic, antibiofilm, and hepatoprotective activities (Atun et al., 2018; Deng et al., 2022; Firmansyah et al., 2023; Jiang et al., 2012; Kanchanapiboon et al., 2020; Martinez-Correa et al., 2017; Miyakoshi et al., 2004; Ongwisespaiboon and Jiraungkoorskul, 2017; Rahmat et al., 2010; Sharma et al., 2022; Singh et al., 2022; Srivastava et al., 2022; Sritananuwat et al., 2024; Tyagi et al., 2015). The remarkable pharmacological properties of these species underscore their enduring importance in traditional medicine and their potential for modern drug development. However, systematic investigations into the chemical profiles and bioactivities of essential oils derived from these plants, particularly those native to high-altitude regions such as the Tenasserim Range in Western Thailand, are scarce.
Detailed studies on the composition and biological activities of essential oils are crucial for advancing their practical applications. Comprehensive research is essential in promoting the sustainable utilization and innovative development of these valuable natural resources.
Essential oils are intricate mixtures of volatile compounds, predominantly terpenes and terpenoids, extracted from plant materials. They are widely recognized for their unique fragrances and extensive therapeutic properties (Asbahani et al., 2015; de Sousa et al., 2023; Raut and Karuppayil, 2014). Methods such as hydrodistillation, steam distillation, and supercritical fluid extraction are employed to maximize yield while preserving bioactive compounds (Tongnuanchan and Benjakul, 2014). In plants, essential oils serve critical ecological functions, such as defending against herbivores and pathogens and facilitating environmental interactions. These oils exhibit a range of biological activities, including antimicrobial, antioxidant, anti-tyrosinase, insect repellent, analgesic, and anti-inflammatory effects, generating considerable interest in the food, agriculture, cosmetic, and pharmaceutical industries (Bilia et al., 2014; de Matos et al., 2019; Herman and Herman, 2015). Headspace solid-phase microextraction (HS-SPME) is an efficient and eco-friendly method for analyzing volatile compounds. This solvent-free technique extracts volatile components directly from the sample’s headspace, preserving their chemical integrity. Paired with gas chromatography-mass spectrometry (GC-MS), HS-SPME offers excellent sensitivity and specificity, providing detailed profiles of essential oils while aligning with sustainable, green chemistry principles (Kowalczyk et al., 2022; Zhao et al., 2023).
The Sufficiency Economy Learning Center in Ban Ta Ko Lang, Ratchaburi Province, leverages the biodiversity of the Tenasserim Range to promote community-driven sustainable development. The organic cultivation of Zingiberaceae plants is a key component of local initiatives to create value-added products such as herbal teas, traditional medicines, and body care products. However, the absence of systematic conservation and utilization strategies restricts the full potential of these resources. From April 2022 to November 2023, our research team surveyed Zingiberaceae species in this area, identifying 6 genera and 36 species, including Curcuma (17 species), Kaempferia (9 species), Boesenbergia (3 species), Globba (3 species), Zingiber (2 species), and Amomum (2 species). A comprehensive Zingiberaceae database was developed, with the establishment of a conservation and educational learning plot to raise community awareness about sustainable plant use and demonstrate how biodiversity conservation can support economic development.
The volatile compounds and bioactive properties of the essential oils extracted from the rhizomes of B. rotunda and C. longa were investigated, focusing on their potential applications in health and beauty. The plants were cultivated in a dedicated plot, using cultivars originally sourced from the Tenasserim Range in Ratchaburi. Our findings provide valuable insights for the sustainable development of value-added products derived from these essential oils, benefiting the local community and expanding their applications across various industries.
2. MATERIALS AND METHODS
2.1. Plant material
Fresh rhizomes of B. rotunda (L.) Mansf. and Curcuma longa L. (Figure 1) were collected in January 2023 from a medicinal herb plantation at the Sufficiency Economy Learning Center, Baan Ta Ko Lang, Suan Phueng District, Ratchaburi Province, Thailand (13°27’05.5”N latitude, 100°00’21.0”E longitude). Preliminary species identification was performed based on morphological characteristics and validated using standard botanical databases. The plant specimens were authenticated by experts at the Herbarium of Mahidol University, Thailand, and deposited under voucher specimen numbers PBM 006426 (B. rotunda) and PBM 006427 (C. longa).
2.2. Isolation and physical characterization of essential oils
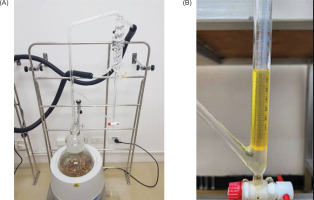
The plant materials were meticulously washed to remove residual soil, sliced into smaller fragments, and dried in a hot air oven at 45°C until their moisture contents were below 10%. A 1 kg aliquot of the dried materials was subjected to hydrodistillation using a Clevenger-type apparatus for 6 hours or until the oil extraction was complete. The essential oils were separated and the yield was calculated as a percentage of the dry weight (w/w). Each extraction was conducted in triplicate to ensure reproducibility. Residual moisture was eliminated by drying the essential oils over anhydrous sodium sulfate. The oils were then transferred to amber glass bottles and stored at 4°C to preserve their stability.
The refractive index of the oils was determined using an Atago PAL-1 handheld refractometer (Tokyo, Japan), and optical rotation measurements were obtained with a Bellingham Stanley ADP 220 polarimeter.
2.3. Volatile composition analysis
Analysis of the volatile compounds in the essential oils was conducted using HS-SPME with DVB-CAR-PDMS (Divinylbenzene/Carboxen/Polydimethylsiloxane) fibers. A 10 µL sample of essential oil was placed in a 20 mL headspace vial, sealed, and incubated at 60°C for 30 minutes to extract the volatiles. The extracted volatiles were then thermally desorbed into a gas chromatograph (GC) injector at 220°C for 10 minutes.
GC analysis was conducted using an Agilent 7890A system coupled with a 5975C quadrupole mass spectrometer (MS) equipped with an HP-5MS column (30 m × 0.25 mm i.d. × 0.25 µm film thickness). The injector and detector temperatures were set at 240°C, operating in split mode with a 1:20 split ratio. Helium was used as the carrier gas at a flow rate of 1 mL/minute. The column temperature program was set from 60°C to 240°C with a heating rate of 3°C/minute and held for 5 minutes at the final temperature. Mass spectra were acquired in electron impact mode (70 eV) over a mass range of 40–550 m/z. Retention indices (RIs) were calculated using a C8–C20 n-alkane series (Sigma-Aldrich) according to the Van den Dool method. Compound identification was performed by comparing retention times and mass spectra with authentic standards and the Wiley11-NIST17 library (Wiley, USA).
2.4. Determination of antioxidant properties
The antioxidant activity of the essential oils was quantified using two complementary radical scavenging assays—DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2’-azino-bis[3-ethylbenzothiazoline-6-sulfonic acid])—following standard protocols with minor adaptations.
2.4.1. DPPH radical scavenging assay
The DPPH assay was performed to evaluate the ability of the essential oils to neutralize free radicals, following the method described by Brand-Williams et al. (1995). Briefly, 50 μL of the essential oil was mixed with 200 μL of 0.1 mM DPPH solution in a 96-well microplate. The reaction mixture was incubated in darkness at ambient temperature for 30 minutes. Absorbance was then measured at 517 nm using a spectrophotometer. Trolox, a water-soluble vitamin E analog, was employed as the reference antioxidant, while the DPPH solution without essential oil served as the negative control. The scavenging activity of each sample was expressed as a percentage, calculated using the equation:
where Acontrol and Asample represent the absorbance of the control and the sample, respectively. The IC50 value, representing the concentration at which 50% of the DPPH radicals were scavenged, was determined through dose–response analysis.
2.4.2 ABTS radical scavenging assay
The ABTS radical scavenging assay was used to evaluate the antioxidant potential of the essential oils following Cai et al. (2004). ABTS (7 mM) was reacted with 2.45 mM potassium persulfate in the dark for 16 hours to generate ABTS•+ radicals. The solution was diluted with ethanol to reach an absorbance of 0.7 ± 0.05 at 734 nm. Essential oil samples (150 μL) were combined with the ABTS•+ solution in a 96-well microplate and incubated for 6 minutes. Absorbance was measured at 734 nm using a spectrophotometer. Trolox was used as the reference antioxidant to facilitate comparison of the scavenging activity and calculated using the same formula as for the DPPH assay. The IC50 value was derived from the dose–response curve via linear regression.
2.5. Antibacterial activity evaluation
The antibacterial properties of the essential oils were evaluated against Escherichia coli (DMST 4212), Pseudomonas aeruginosa (DMST 4739), and Staphylococcus aureus (DMST 8840). These bacterial strains were sourced from the Department of Medical Sciences, Ministry of Public Health, Thailand, and cultured on nutrient agar slants. Bacterial suspensions were standardized to 108 colony-forming units (CFU)/mL before experimentation.
The essential oil samples were dissolved in dimethyl sulfoxide (DMSO) and sterilized using a 0.45 µm syringe filter. Serial twofold dilutions were then prepared in nutrient broth to obtain a spectrum of concentrations. The antibacterial efficacy was assessed using a resazurin-based microdilution assay, following the protocol established by Sarker et al. (2007). Each dilution was introduced into a 96-well microplate containing the standardized bacterial suspension. Negative controls consisted solely of bacterial suspensions, whereas positive controls included nutrient broth and essential oil solutions. Following incubation at 37°C for 24 hours, resazurin solution was added as an indicator of bacterial viability. A shift in color from blue to pink or clear denoted bacterial growth. The minimum inhibitory concentration (MIC) was identified as the lowest concentration preventing any color change. To determine the minimum bactericidal concentration (MBC), aliquots from wells exhibiting no visible growth were plated onto sterile agar media. The MBC was recorded as the lowest concentration that entirely inhibited bacterial proliferation. Erythromycin was utilized as the standard antibiotic control for comparative analysis.
3. RESULTS AND DISCUSSION
3.1. Isolation of essential oils, physical properties, and volatile composition analysis
The yields and physical properties (refractive indices and specific rotations) of B. rotunda and C. longa essential oils via hydrodistillation (Figure 2) are summarized in Table 1. Both oils exhibited a clear yellowish color, with yields ranging from 1.02% to 1.52% based on dry weight. The oil yield of C. longa was higher than B. rotunda. Volatile compounds in the essential oils were analyzed using HS-SPME coupled with GC-MS, as detailed in Table 2.
Table 1
Yield (%), refractive index, and specific rotation of B. rotunda and C. longa essential oils.
| Essential oils | Yield (%)* | Refractive index (RI) | Specific rotation |
|---|---|---|---|
| B. rotunda C. longa | 1.02 ± 0.26 1.52 ± 0.07 | 1.48 1.51 | 71.95 66.63 |
Table 2
Relative contents, retention indices (RIs), and volatile compositions of B. rotunda and C. longa essential oils.
Thirty-one volatile compounds were identified in the essential oil of B. rotunda while 44 were detected in C. longa. The top three primary constituents in B. rotunda were camphor (23.28%), 1,8-cineole (15.67%), and β-cis-ocimene (14.79%) while C. longa recorded ar-turmerone (27.91%), α-terpinolene (14.16%), and 1,8-cineole (12.32%). These essential oils displayed diverse chemical profiles, with significant variability attributed to regional and environmental factors.
The compositions of B. rotunda reported by various research groups emphasized regional differences. Apinundecha et al. (2023) found 24 compounds in essential oil from Northeastern Thailand with the primary constituents β-ocimene (36.73%), trans-geraniol (25.29%), and camphor (14.98%). Liana et al. (2024) identified 14 compounds from Thailand, with camphor (35.25%) as the major component, followed by 1,8-cineole (20.47%), and nerol (13.86%). Baharudin et al. (2015) found 18 compounds in oil from Malaysia, with nerol (39.6%) and camphor (36.0%) as the dominant constituents. Kurnia et al. (2024) identified 10 compounds in oil from Central Java, Indonesia, with camphor (28.29%) and 1,8-cineole (27.13%) as the major components. Research indicated that essential oils with camphor as a major constituent exhibited antimicrobial, anticancer, cough suppressant, and transdermal absorption-enhancing properties (Chen et al., 2013). Camphor also showed promise in supporting therapy for skin infections with biological activities, including anti-inflammatory, antibacterial, antifungal, anti-acne, anesthetic, strengthening, and warming effects as an effective agent for preventing dermatological infectious diseases and a key component in medical and cosmetic products (Abdollahi et al., 2020; Duda-Madej et al., 2024; Kang et al., 2019; Nurzyńska-Wierdak et al., 2023).
The compositions of C. longa reported by different research groups also showed regional variations. Brazilian essential oils predominantly contained ar-turmerone (40.00–42.6%), α-turmerone (10.05–42.6%), and curlone (12.9–22.73%) (Avanço et al., 2017; Guimarães et al., 2020). In Nigeria, ar-turmerone (35.90%), α-phellandrene (15.50%), and curlone (12.90%) were identified as the dominant compounds (Oyemitan et al., 2017). Oils from the Indo-Gangetic Plains showed high levels of α- and β-turmerones (40.8%), with notable amounts of myrcene and 1,8-cineole (Bansal et al., 2002). Similarly, North Alabama oils contained ar-turmerone (6.8–32.5%), α-turmerone (13.6–31.5%), β-turmerone (4.8–18.4%), α-phellandrene (3.7–11.8%), and 1,8-cineole (Setzer et al., 2021). The primary bioactive constituents (ar-turmerone, α-turmerone, and β-turmerone) consistently exhibited antimicrobial, antioxidative, anti-inflammatory, and anticancer properties (Meng et al., 2018). These compounds are widely used in pharmaceutical and cosmetic applications. Ar-turmerone, a key sesquiterpene, is recognized for its potent anticancer effects, with distinct mechanisms of action observed in various cancer cell lines (Liu et al., 2016; Nair et al., 2019; Yue et al., 2016). Zheng et al. (2020) identified 56 components in C. longa oil from China, with ar-turmerone (36.04%) as the predominant compound. Their study highlighted the protective effects of ar-turmerone against ultraviolet B radiation (UVB)-induced photoaging, underscoring its potential for use in skincare and cosmetic formulations.
3.2. Determination of antioxidant properties
The antioxidant activities of B. rotunda and C. longa essential oils were investigated using DPPH and ABTS radical scavenging assays, recognized for their accuracy, rapid results, and reproducibility in assessing antioxidant potential. The IC50 values, representing the concentrations required to neutralize 50% of DPPH and ABTS radicals, are summarized in Table 3. These values provided a quantitative measure of antioxidant strength, with lower IC50 values indicating stronger antioxidant activity. Results showed that both essential oils exhibited significant antioxidant activity, with C. longa demonstrating superior antioxidant efficiency compared to B. rotunda and Trolox in both assays. The B. rotunda oil exhibited higher antioxidant activity than Trolox in the ABTS assay but lower activity than Trolox in the DPPH assay.
3.3. Antibacterial activity evaluation
The antibacterial properties of essential oils from C. longa and B. rotunda were assessed against three bacterial strains: 2 g negative strains (E. coli DMST 4212 and P. aeruginosa DMST 4739) and 1 g positive strain (S. aureus DMST 8840) commonly found in hospitalized patients. Results demonstrated that C. longa essential oil only inhibited the growth of S. aureus, with MIC and MBC values of 250 mg/mL. In contrast, B. rotunda essential oil exhibited broader antibacterial activity, effectively inhibiting all three bacterial strains, with MIC values ranging from 31.25 to 250 mg/mL. The MBC for S. aureus was 62.50 mg/mL, summarized in Table 4.
Table 4
Minimal inhibitory concentration (MIC, mg/mL) and minimal bactericidal concentration (MBC, mg/mL) of B. rotunda and C. longa essential oils and the standard erythromycin against the tested bacterial strains.
| Essential oil/Standard | E. coli | S. aureus | P. aeruginosa | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| B. rotunda C. longa Erythromycin | 125 - 0.01 | - - 0.01 | 31.25 250 0.01 | 62.50 250 0.01 | 250 - 3.13 | - - 3.13 |
These findings suggested that both C. longa and B. rotunda essential oils exhibited limited antibacterial activity against the tested strains compared to the standard antibiotic erythromycin. Plant-derived essential oils have significant potential as safe and natural alternatives for antimicrobial development, and the application of advanced technologies to enhance their efficacy in antimicrobial treatments is of great interest and can offer substantial benefits.
4. CONCLUSIONS
This study identified 31 volatile compounds in the essential oil of B. rotunda and 44 in C. longa, emphasizing their chemical diversity and potential applications. The C. longa essential oil, with a yield of 1.52%, was primarily composed of ar-turmerone (27.91%), α-terpinolene (14.16%), and 1,8-cineole (12.32%) demonstrating significant antioxidant activity (IC50: 10.98 mg/mL by DPPH and 6.11 mg/mL by ABTS), and suggesting its potential for anti-aging, skincare, and health benefits. In contrast, B. rotunda yielded 1.02%, with camphor (23.28%), 1,8-cineole (15.67%), and β-cis-ocimene (14.79%) as the major components. The B. rotunda antioxidant activity was lower than C. longa but exhibited a broader spectrum of antibacterial activity, inhibiting E. coli, P. aeruginosa, and S. aureus. Both oils showed lower antibacterial effectiveness compared to erythromycin. Our findings suggested that advanced methods such as nanoencapsulation could enhance the antibacterial properties of these oils and increase their potential for wider applications.
This study provides a comprehensive analysis of the chemical compositions and bioactivities of B. rotunda and C. longa essential oils sourced from the Tenasserim Range in Western Thailand. Our findings offer valuable insights into the potential applications of these oils in health and beauty products. The knowledge and technologies developed through this research will be shared with local communities to promote sustainable practices. Future studies will prioritize the formulation of personal care products derived from these plant extracts, with an ongoing emphasis on community engagement and sustainable development.