1. INTRODUCTION
Seaweeds, also known as marine macroalgae, are significant biological resources in ocean habitats and are usually found on stable substrates such as rocks and shells. Seaweeds are marine vegetables that have traditionally been consumed in several Asian countries. They preserve coastal and aquatic biodiversity and offer important ecological services such as storm protection, food, and medicinal resources (Sahayaraj et al., 2014). According to recent research, macroalgae are highly sought-after as a natural source of bioactive compounds with diverse biological activities. Numerous researchers from throughout the world have discussed the therapeutic values of seaweeds of the Chlorophyceae, Phaeophyceae, and Rhodophyceae families (Kolanjinathan et al., 2009).
It has been demonstrated that extracts from a wide range of macroalgae in almost every group and location possess antibacterial properties. In the marine environment, secondary metabolites of algae with antibacterial qualities may serve as active defence mechanisms against epiphytons and maintain their ability to quickly recover and regenerate after being damaged by abrasions or predators (Vlachos et al., 2001). Besides being expensive, synthetic drugs often have side effects and are tampered with. Thus, it is necessary to find new approaches to manage microbial infections. Compounds from plants, soil, and other sources, including marine species, are becoming more and more important to the pharmaceutical industry (Sieradzki et al., 2003).
Plant-based antioxidants, such as Vitamin C and carotenes, protect cells from damaging free radicals that cause inflammation and cancer (Palpperumal et al., 2024). Biofilms are microbial sessile communities embedded in self-secreted extracellular matrices that are affixed to a substrate, interfaces, or one another. Natural materials gained appeal
due to the significance of biofilm and worries about its severe issues. Furthermore, evaluating the antibiofilm properties of their derivatives is essential, given the possibility of using algae as a novel biological agent (Muras et al., 2021).
Microorganisms such as bacteria, diatoms, and fungi colonize aquatic environments, and macro-organisms such as barnacle larvae or spores, seaweed, and invertebrates settle on both natural and man-made substrates. This process is known as biofilm. Usually, biofilm is the first step in fouling, caused by bacteria adhering to surfaces extremely tightly due to forces of attraction and repulsion. This layer is the base for the attachment and development of other foulers (Wigglesworth-Cooksey & Cooksey, 2005). Reversible physical interactions, such as electrostatic contact and water movement, cause biofilm growth throughout the biofouling process. Therefore, preventing the production of biofilms is a crucial step in stopping the fouling process (Cao et al., 2011). Marine biota, such as algae, invertebrates, seagrasses, sponges, and ascidians, are the finest alternatives in the most efficient antifouling processes. They generate a variety of bioactive substances with antifouling properties (Prabhakaran et al., 2012).
Understanding emulsifying properties and stability is crucial when creating a range of food products, including salad dressings, dairy products, and sauces, to obtain texture, quality, and longer shelf life. To allow two nonmiscible liquids to mix as small droplets, emulsions are made by reducing their surface tension. The ability of the emulsion to tolerate alterations over time, such as problems like creaming and coalescence, determines the stability and longevity of emulsified goods. This characteristic is termed “emulsion stability.” Reports state that seaweed has a remarkable capacity for emulsification (Bajad et al., 2024).
Hydrocolloids are high molecular weight polysaccharides that are connected to the cell walls and intercellular spaces of seaweeds. These polymers act as the primary structural component of cell walls and participate in the mechanisms that identify pathogens (Cardozo et al., 2007). According to Pangestuti and Kim (2014), algal polysaccharides provide structural functions like stiffness, accumulate mucilage in their cell walls, and protect against stress and desiccation. These phycocolloids have a large water-holding capacity, which enables them to chelate and gel metals. In addition, they have been used commercially in various industries, including food technology, engineering, pharmaceutical sciences, biotechnology, and cosmetics.
Caulerpa scalpelliformis (Turner) C. Agardh is a seaweed species of the Caulerpaceae family. Straight fronds that are around 3 cm (1.18 in) broad, this epilithic green seaweed usually reaches a height of 20 cm (7.9 in). In rocky waters, they can be found in rock pools up to 30 m (98 feet) deep. In Tamil Nadu, it is commonly found in the coastal areas of Thoothukudi region (Mahadevi & John 2014). Numerous studies have confirmed the antibacterial and antioxidant properties of seaweed (Chandini et al., 2008). The current investigation evaluated their antimicrobial, antioxidant, antibiofilm, and emulsifying properties. GC-MS analysis of various phytoconstituents present in the sulfated polysaccharides extracted from C. scalpelliformis.
2. MATERIALS AND METHODS
2.1. Description of the study area
There are 19 islands in the Gulf of Mannar Marine Biosphere Reserve, which stretches from Rameswaram to Kanyakumari (Longitudes 78° 08’ – 79° 30’ E and Latitudes 8° 35’ – 9° 25’ N) in the southeast coast of Tamil Nadu, India, with around 212.6 km in length. Seaweed samples were gathered from the shores of the Gulf of Mannar Marine Biosphere Reserve mainland. The 19 islands in the Gulf of Mannar are home to many seaweeds, with around 147 different kinds of marine algae. Palpperumal et al. (2024) documented 69 red, 42 green, 31 brown, and 5 blue–green algae in the study area. The collected samples were taxonomically identified by referring to the book by Murugaiyan and Anantharaman (2019), and herbarium was deposited in the PG and Research Department of Botany, Saraswathi Narayanan College, Madurai, India.
2.2. Extraction of crude polysaccharide from C. scalpelliformis
Polysaccharides were extracted from dried C. scalpelliformis using the technique by Ananthi et al. (2010). One gram of C. scalpelliformis dried biomass was added to 50 mL distilled water, and the mixture was incubated at room temperature for 30 min before being refluxed at 90 °C and stirred at 400 rpm for 1, 2, 3, or 4 h and extracted using autoclaving (121 °C, 30 min). To improve the extraction efficiency, pellet reflux was done twice after chilling, and centrifugation was used to recover the supernatant. After that, the extracts were mixed, concentrated, and cooled to 4 °C. After the addition of three volumes of ethanol, the mixture was centrifuged and pellets (total polysaccharides extract) were lyophilized and stored at −80 °C for future use.
2.3. Estimation of total carbohydrates of polysaccharides
The total carbohydrate content was estimated using the phenol sulphuric acid method (Dubois et al., 1956). The total carbohydrate in the sample was expressed as a percentage using Equation 1.
2.4. Antibacterial assay of sulfated polysaccharides from Caulerpa scalpelliformis
2.4.1. Target microorganisms
Seven distinct human pathogenic strains were used for the in vitro antibacterial susceptibility assay. They included Proteus mirabilis (ATCC 29906), Streptococcus pneumoniae (ATCC 33400), Escherichia coli (ATCC 25922), Salmonella typhi (ATCC 6539), Staphylococcus aureus (ATCC 25923), Bacillus subtilis (ATCC 6051), and Pseudomonas aeruginosa (ATCC 27853). All strains were kept in nutrient agar slants.
2.4.2. Inoculum preparation
The nutrient broth was prepared in test tubes and autoclaved for 15 min at 15 lbs pressure. The sterilized broth was used to individually inoculate each of the seven bacterial strains, which were then cultured for 24 h at 37 °C.
2.4.3. Well diffusion method
0.1 mL of the 24-hour-old culture of bacterial strains was added to sterile Mueller–Hinton agar plates. 4 mm wells were created using a sterile cork borer in a petri dish. Each well received 20 μL of diluted extract in 100 μL of sterile distilled water, and 10 μL of ampicillin was added to the control well. Following 1 day of incubation at 37 °C, the antibacterial activity was estimated by measuring the zone of inhibition on the plates in millimeters (Palpperumal et al., 2024).
2.5. Molecular docking
The LigPrep module was used to preprocess the 3D structures of bioactive compounds that were obtained from the PubChem database, producing 32 distinct stereoisomers computationally. The energy was minimized using the OPLS4 force field (Schrödinger Release 2024-4: LigPrep.) The Protein Preparation Wizard module was used to preprocess proteins, including methionine conversion, hydrogen addition, side chain filling, metal bond formation, and bond order assignment. Finally, the force field from OPLS4 was used. Additionally, the Ligand Docking module was used to dock the preprocessed ligands and macromolecules. The structural interpretations, bond length, and interaction were recorded.
2.6. Antibiofilm effect of sulfated polysaccharides from Caulerpa scalpelliformis
2.6.1. Crystal violet staining method
The antibiofilm impact of sulfated polysaccharides was investigated using the crystal violet staining method on a 24-well polystyrene (hydrophobic) microtiter plate (Stepanovic et al., 2007).
2.6.2. Ring biofilm assay
The study investigated the impact of sulfated polysaccharides on ring biofilm formation at the air–liquid interface by cultivating MRSE in hydrophilic glass test tubes (Chavant et al., 2007).
2.7. Antioxidant activity of sulfated polysaccharides from Caulerpa scalpelliformis
2.7.1. Evaluation of Total Antioxidant Capacity (TAC)
The antioxidant capacity of C.x scalpelliformis polysaccharides was calculated using the method by Prieto et al. (1999). Ascorbic acid was used as the standard.
2.7.2. Evaluation of reducing power
The technique by Yamaguchi et al. (1998) was used to check the reducing power of sulfated polysaccharides extracted from C. scalpelliformis. Ascorbic acid was used as the standard.
2.7.3. Hydrogen peroxide radicals scavenging activity
The hydrogen peroxide radical scavenging activity of sulfated polysaccharides derived from C. scalpelliformis was analyzed using the method of Gulcin et al. (2004). Gallic acid was used as the standard, and the percentage of H2O2 scavenging was determined using Equation 2.
Where A0 is the Absorbance of the control, and A1 is the Absorbance of the sample.
2.7.4. DPPH radical scavenging assay
The DPPH (1,1-diphenyl-2-picrylhydrazyl) was prepared using the method by Blois (1958) to evaluate the DPPH radical scavenging activity of sulfated polysaccharides from C. scalpelliformis. Gallic acid was used as the standard, and the percentage of sulfated polysaccharide free radical scavenging activity was determined using Equation 3.
Where A0 is the absorbance of the control, and A1 is the absorbance of the sample turbidity factor.
2.7.5. Superoxide anion radical scavenging assay
The superoxide anion scavenging activity of sulfated polysaccharides was evaluated using the method of Nishikimi et al. (1972). Gallic acid was used as the standard, and the percentage of free radical scavenging was determined using the following formula (Equation 4).
Where A0 is the Absorbance of the control, and A1 is the Absorbance of the sample.
2.7.6. Nitric oxide radical scavenging assay
The Griess reaction was used to detect nitrite ions from nitric oxide radicals in a sodium nitroprusside solution at physiological pH (Gulcin, 2006). Gallic acid was used as the standard, and the percentage of free radical scavenging activity was determined using the following formula (Equation 5).
Where A0 is the Absorbance of the control, and A1 is the Absorbance of the sample.
2.8. Emulsification activity of sulfated polysaccharides from Caulerpa scalpelliformis
The emulsifying activity of sulfated polysaccharides was measured using the Cooper and Goldenberg (1987) technique. This approach was used to assess the emulsification principle of the samples. Oil or hydrogen was added to the samples to prepare the aqueous phase.
2.9. HPLC analysis of sulfate polysaccharides
The sulfated polysaccharides were analyzed using a high-performance liquid chromatography (HPLC) C18 system column (LC-10VP Shimadzu) and eluted with distilled water at a flow rate of 1.0 mL/min at 20 °C. The separated components were monitored using a refractive index detector. The sulfated polysaccharides were hydrolyzed, and derivatives with methanol were analyzed for their sugar composition by HPLC. The column was calibrated with different molecular mass standards and a standard curve.
2.10. Gas chromatography–mass spectrometry analysis
The gas chromatography–mass spectrometry (GC-MS) study of sulfated polysaccharides was carried out using a Shimadzu GC-MS-QP 2010 ultra-gas chromatographic system. 10 µg/mL of extract was analyzed on a capillary column (BPX5: 5% phenyl, 95% methyl polysilphenylene, 30 m × 0.25 μm × 0.25 μm). The column temperature program was set at 50 °C with a temperature increase of 3 °C/min to 300 °C (10 min), whereas the injector temperature was set at 250 °C. Pure helium gas (99.999%) with a flow rate of 0.8 mL/min was utilized as the carrier gas, and a split ratio of 1:10 was employed. Electron ionization (EI) was the ion source with a temperature of 200 °C, and an ionization voltage of 70 eV was employed with a detecting voltage of 0.87kV. The interface temperature was 250 °C, and the solvent cutoff time was 2 min. The process was scheduled to start at 2.5 min and conclude at 93 min. The interpretation of the mass spectrum of GC-MS was done using the National Institute of Standards and Technology (NIST) database, which has over 62,000 patterns. The mass spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST library (NIST, 2011).
3. RESULTS
3.1. Estimation of total carbohydrates
The phenol sulphuric acid method was used to estimate the total carbohydrates present in polysaccharides. Glucose was used as the standard, and the percentage of total carbohydrates was found to be 68.15 ± 0.45.
3.2. Antibacterial activity of sulfated polysaccharides from Caulerpa scalpelliformis
The antibacterial efficacy of C. scalpelliformis–isolated sulfated polysaccharides against seven distinct human pathogens, namely P. mirabilis, S. pneumoniae, E. coli, S. typhi, S. aureus, B. subtilis, and P. aeruginosa, was assessed, and the results are presented in Table 1. The highest activity was seen against P. aeruginosa, with an inhibition zone of 18 mm, while the minimum antibacterial activity was recorded against P. mirabilis. When compared to the polysaccharides of C. scalpelliformis, the conventional Ampicillin exhibited reduced antibacterial effectiveness but had notable inhibitory action (Table 1).
Table 1
Antibacterial activity of sulfated polysaccharides from Caulerpa scalpelliformis.
3.3. Antibiofilm activity of sulfated polysaccharides from Caulerpa scalpelliformis
3.3.1. Crystal violet staining method
The microtiter plate was examined using the crystal violet staining procedure. The biofilm formation was measured using the microtiter plate test, and the percentage of biofilm inhibition by B. subtilis in response to varying concentrations of sulfated polysaccharides was also investigated. The majority of the results demonstrated that administering subinhibitory doses of sulfated polysaccharide extracts to cells considerably decreased the production of biofilms in a dose-dependent manner. The sulfated polysaccharides exhibited 60.12 ± 0.3% inhibition of biofilm development by B. subtilis at the maximum concentration tested (100 μg/mL) (Table 3).
Table 2
The molecular docking of bioactive compounds with multiple antibacterial target proteins shows that the lowest binding score has the highest binding affinity.
3.3.2. Ring biofilm assay
The ring biofilm assay is a tube method and a qualitative assay for detecting biofilm development. The percentage inhibition of biofilms in B. subtilis in response to varied amounts of sulfated polysaccharides was also studied using this assay. The results indicated a lack of biofilm production; the sulfated polysaccharides (25, 50, 75, and 100 μL) gradually reduced biofilm formation. They exhibited 24.56 ± 0.065% of inhibition of biofilm formation by B. subtilis at the hi ghest concentration subjected (100 μg/mL) (Table 4).
3.4. Antioxidant activity of sulfated polysaccharides of Caulerpa scalpelliformis
3.4.1. Total antioxidant capacity
The total antioxidant capacity of C. scalpelliformis sulfated polysaccharides and standard ascorbic acid is shown in Table 5. The inhibition percentage of total antioxidant capacity exhibited a concentration-dependent pattern. The sulfated polysaccharides had higher antioxidant capacity at 1000 μg/mL (90.15 ± 0.45%), followed by 750 μg/mL (79.21 ± 0.15%) concentration. The least inhibition percentage (46.16 ± 0.11%) was observed with a low concentration of sulfated polysaccharides (100 μg/mL). Likewise, ascorbic acid showed higher antioxidant activity of 95.22 ± 0.17% at 1000 μg/mL and lowest (51.21 ± 0.12%) at 100 μg/mL concentration (Table 5).
3.4.2. Reducing power assay
Table 6 highlights the results of the reducing power test using the standard ascorbic acid and sulfated polysaccharides extracted from C. scalpelliformis. Sulfated polysaccharides from C. scalpelliformis are compared to the standard ascorbic acid. The reducing power was 1.05 ± 0.03%. The increase in the concentrations of sulfated polysaccharides from C. scalpelliformis was shown to enhance the reducing power.
3.4.3. Hydrogen peroxide scavenging assay
Table 7 highlights the hydrogen peroxide scavenging capacity of C. scalpelliformis. The sulfated polysaccharides and standard gallic acid at various concentrations (100, 250, 500, 750, and 1000 μg/mL) were compared. The scavenging percentage exhibited a concentration-dependent antioxidant capacity. The sulfated polysaccharides had the highest nitric oxide scavenging ability at 1000 μg/mL (80.25 ± 0.04%), followed by 750 μg/mL (69.15 ± 0.05%) concentration. The least inhibition percentage (40.27 ± 0.15%) was observed with a low concentration of sulfated polysaccharides (100 μg/mL).
3.4.4. DPPH radical scavenging activity
The ability of standard gallic acid and C. scalpelliformis sulfated polysaccharides to scavenge DPPH radicals at different concentrations (100, 250, 500, 750, and 1000 μg/mL) is emphasized in Table 8. The inhibition percentage of DPPH revealed a concentration-dependent antiradical scavenging activity, and the activities exhibited by the sulfated polysaccharides in this study were low when compared to that of gallic acid. In sulfated polysaccharides, the DPPH reduction capacity was highest at 1000 μg/mL (89.06 ± 0.41%), followed by 750 μg/mL (78.40 ± 0.15%) concentration. The least inhibition percentage (44.42 ± 0.16%) was observed with a low concentration of sulfated polysaccharides (100 μg/mL). Likewise, gallic acid showed the highest inhibition percentage (95.19 ± 0.41%) at 1000 μg/mL and the lowest activity (48.12 ± 0.15%) at 100 μg/mL concentration level (Table 8).
3.4.5. Superoxide anion radical scavenging activity
The current study showed that the maximum superoxide anion free radical scavenging activity was detected in sulphated polysaccharides against standard gallic acid (69.17 ± 0.42%) at 1000 µg/mL concentration. It was followed by values 58.43 ± 0.11% and 49.19 ± 0.28% at 750 and 500 µg/mL concentrations, respectively. Furthermore, the gallic acid exhibited 78.33 ± 0.15% and 36.21 ± 0.14% as maximum and minimum superoxide anion radical scavenging activity of C. scalpelliformis, respectively (Table 9).
3.4.6. Nitric oxide scavenging activity
The nitric oxide scavenging activity of sulfated polysaccharides derived from C. scalpelliformis is presented in Table 10. The sulfated polysaccharides exhibited decreased scavenging activity (39.36 ± 0.42%) at lower concentration (100 µg/mL) and maximum activity (75.15 ± 0.45%) at 1000 µg/mL concentration (Table 10).
3.5 Emulsifying activity of sulfated polysaccharides of Caulerpa scalpelliformis
The ability of C. scalpelliformis to emulsify various oils and hydrocarbons was investigated in the present study. In case of different oils, high emulsification index of sulfated polysaccharides was observed in gingelly oil (270 ± 0.5%), followed by olive (269 ± 0.5%), coconut (266 ± 0.5%), palm (266.6 ± 0.5%), sunflower (237.7 ± 0.5%), groundnut (253 ± 0.5%), and castor (243.3 ± 0.5%) oils. Among different hydrocarbons, the emulsification index is in the order of diesel (267 ± 0.5%) > kerosene (240 ± 0.5%) > petrol (198 ± 05%) > Tween 20 (100 ± 0.5%).
3.6 HPLC analysis
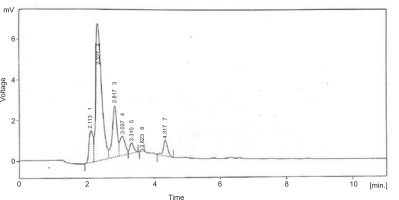
The purified sulfated polysaccharides were examined by HPLC system with C18 column and gained six peaks. The peaks indicated the presence of neutral sugars such as ribose (2.113 and 2.327), fructose (2.817), rhamnose (3.037 and 3.310), and mannose (3.623 and 4.317) as shown in Figure 5.
Figure 1
The two-dimensional interactions of (A) PORE COAT POLYSACCHARIDE BIOSYNTHESIS PROTEIN SPSA – 2,3,5,6-tetrafluoro-4-methoxybenzoic acid; (B) A6QHR2 – Phenol; (C) 1QU3 – Phenol; (D) ISOLEUCYL-TRNA SYNTHETASE – 2,3,5,6-tetrafluoro-4-methoxybenzoic acid; (E) ycgJ – 2,3,5,6-tetrafluoro-4-methoxybenzoic acid; (F) PROCESSED GLYCEROL PHOSPHATE LIPOTEICHOIC ACID SYNTHASE 2 – Phenol.
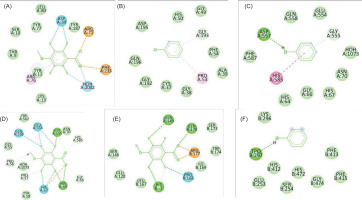
Figure 2
The two-dimensional interactions of (A) Dihydrofolate reductase – Phenol; (B) 3SRW – 2,2,4,4,6,6-hexamethyl-1,3,5,2,4,6-trioxatrisilinane; (C)Dihydrofolate reductase –[dimethyl(trimethylsilyloxy)silyl]oxy-[[[[dimethyl(trimethylsilyloxy)silyl]oxy-dimethylsilyl]oxy-dimethylsilyl]oxy-dimethylsilyl]oxy-dimethylsilane ; (D) Dihydrofolate reductase – 2,3,5,6-tetrafluoro-4-methoxybenzoic acid; (E) Staphylococcus aureus subsp. aureus MW2 – [dimethyl(trimethylsilyloxy)silyl]oxy-[[[[dimethyl(trimethylsilyloxy)silyl]oxy-dimethylsilyl]oxy-dimethylsilyl]oxy-dimethylsilyl]oxy-dimethylsilane; (F) Pseudomonas aeruginosa UCBPP-PA14 - Phenol; (G) Pseudomonas aeruginosa UCBPP-PA14 – 2,3,5,6-tetrafluoro-4-methoxybenzoic acid.
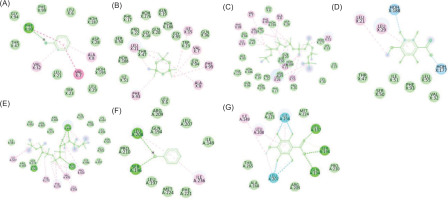
Figure 3
The two-dimensional interactions of (A) SPORE COAT POLYSACCHARIDE BIOSYNTHESIS PROTEIN SPSA – 2,3,5,6-tetrafluoro-4-methoxybenzoic acid; (B) A6QHR2 – Phenol; (C) ISOLEUCYL-TRNA SYNTHETASE – Phenol; (D) ISOLEUCYL-TRNA SYNTHETASE – 2,3,5,6-tetrafluoro-4-methoxybenzoic acid; (E) ycgJ – 2,3,5,6-tetrafluoro-4-methoxybenzoic acid; (F) PROCESSED GLYCEROL PHOSPHATE LIPOTEICHOIC ACID SYNTHASE 2 – Phenol.
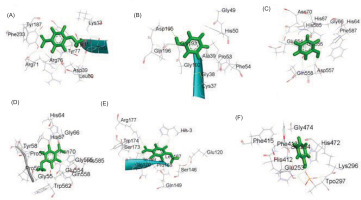
Figure 4
The two-dimensional interactions of a) Dihydrofolate reductase - Phenol; (B) 3SRW – 2,2,4,4,6,6-hexamethyl-1,3,5,2,4,6-trioxatrisilinane; (C) Dihydrofolate reductase – [dimethyl(trimethylsilyloxy)silyl]oxy-[[[[dimethyl(trimethylsilyloxy)silyl]oxy-dimethylsilyl]oxy-dimethylsilyl]oxy-dimethylsilyl]oxy-dimethylsilane ; (D) Dihydrofolate reductase – 2,3,5,6-tetrafluoro-4-methoxybenzoic acid; (E) Staphylococcus aureus subsp. aureus MW2 – [dimethyl(trimethylsilyloxy)silyl]oxy-[[[[dimethyl(trimethylsilyloxy)silyl]oxy-dimethylsilyl]oxy-dimethylsilyl]oxy-dimethylsilyl]oxy-dimethylsilane; (F) Pseudomonas aeruginosa UCBPP-PA14 - Phenol; g) Pseudomonas aeruginosa UCBPP-PA14 – 2,3,5,6-tetrafluoro-4-methoxybenzoic acid.
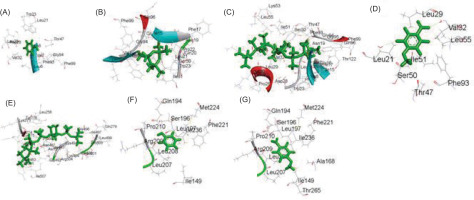
3.7 GC-MS analysis
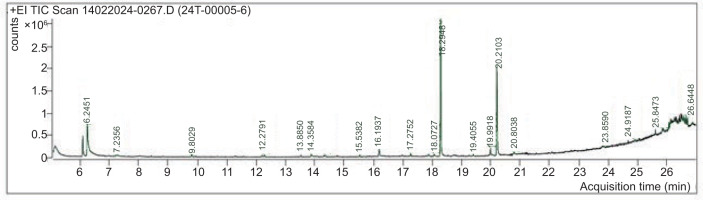
The GC-MS investigation revealed that C. scalpelliformis sulfated polysaccharides are extremely complex. After hydrolysis of sulfated polysaccharides, the mass spectra of the resulting alditol acetates showed electron impact fragmentation patterns. The partly purified product showed the following peaks: 6.2451 (Phenol), 7.2356 (Cyclotrisiloxane, hexamethyl), 9.8029 (Cyclotetrasiloxane, octamethyl), 12.2791 (1,1,3,3,5,5,7,7-Octamethyl-7-[2-methylpropoxy]tetrasiloxan-1-ol), 13.8850 (Dodecanoic acid, methyl ester), 14.3584 (1,1,1,5,7,7,7-Heptamethyl-3,3-bis(trimethylsiloxy)tetrasiloxane), 15.5382 (Cyclooctasiloxane, hexadecamethyl), 16.1937 (Methyl tetradecanoate), 17.2752 (Cyclononasiloxane, octadecamethyl), 18.0727 7 (-Hexadecenoic acid, methyl ester, [Z]-), 18.2948 (Hexadecanoic acid, methyl ester), 19.4055 (Heptasiloxane), 19.9918 (9-Octadecenoic acid), 20.2103 (Methyl stearate), 20.8038 (Octasiloxane), 23.8590 (Octasiloxane), 24.9187 (15-hexadecamethyl), 25.8473 (2,3,5,6-Tetrafluoro-4-methoxybenzoic acid), and 26.6448 (Octasiloxane) as shown in Figure 6. The list of components identified from the sulfated polysaccharides of C. scalpelliformis is given in Table 11 with their respective molecular formula and molecular weight.
Table 11
List of phytocompounds identified from sulfated polysaccharides of Caulerpa scalpelliformis by GC-MS.
4. DISCUSSION
The current study findings were consistent with those of previous research projects. Mrinalini (2014) found that the extracts of C. scalpelliformis showed the highest antibacterial activity against E. coli in methanol, petroleum ether, and chloroform. Kotteswari et al. (2015) examined the green algal C. scallopiformis methanolic extract for in vitro antibacterial activities and found that the growth of all tested microorganisms, particularly B. subtilis and S. aureus, were inhibited at a concentration of 100 μg/mL. In addition to these findings, other investigations revealed that different extracts derived from various species of Caulerpa exhibited potent antibacterial activity against the tested pathogens. In the research conducted by Abdalla et al. (2016), the methanolic extract of Caulerpa racemosa demonstrated an inhibitory zone of 20.0, 16.0, and 19.0, 16.0 mm against gram-negative E. coli and P. aeruginosa.
The outcomes of the present study were also inconsistent with some earlier studies. According to Sasikala and Geetha Ramani (2017), the C. scalpelliformis extract against some bacteria, particularly E. coli, and P. aeruginosa. The season in which the samples were obtained, the extraction techniques, and the solvents employed can contribute to the difference in antibacterial activity. The antimicrobial activity of C. scalpelliformis against harmful bacteria might be a sign of a broad range of antibiotic compounds or the presence of pharmacologically active components such tannins, alkaloids, and saponins (Karthick et al., 2014).
As per earlier reports, algae have been described as renewable sources of bioactive substances, specifically polysaccharides and carotenoids, due to their biological functions, including anti-inflammatory, antimicrobial, antioxidant, anticancer, and anti-Alzheimer effects (Oliyaei et al., 2023). According to some studies, their metabolites or extracts may prevent the production of biofilms (Zammuto et al., 2022). Marine and algal bioactive compounds were indicated as being frequently utilized to prevent and treat bacterial biofilms, among other natural products (Melander et al., 2020). These findings strongly agree with the present study results. Notably, the antibacterial action of sulfated polysaccharides was mainly shown in their capacity to stop biofilms from growing on attached surfaces (Junter et al., 2016).
Gazali et al. (2022) evaluated the antioxidant potential of C. racemosa using a reducing power test. According to Susilowati et al. (2019), polyphenol molecules are part of the bioactive component of C. racemosa. Their antioxidant properties are due to the presence of the hydroxyl groups para to -OH and -OR groups that are substituted in ortho locations. The gallic acid showed the highest scavenging activity (89.14 ± 0.11%) at 1000 μg/mL and the lowest activity (48.16 ± 0.07%) at 100 μg/mL concentration (Table 7). A few variables, including the type, purity, structure, sidechain, molecular weight, and attached groups were shown to affect the antioxidant activity of sulfated polysaccharides of marine algae (Sun et al., 2014).
Numerous studies have focused on the antioxidant potential of Caulerpa species. According to Gazali et al. (2022), the antioxidant value of C. racemosa extracts is in the following order: n-hexane (15.50 ± 0.70 μmoltrolox/g) > ethanol (358±1.41 μmoltrolox/g) > ethyl acetate (568±7.07 μmoltrolox/g). Dissanayake et al. (2022) evaluated the antioxidant capability of C. racemosa using the DPPH test. The results showed an IC50 value of 164.83 μg/mL, which was higher than the IC50 value for the positive control, ascorbic acid (49.88 μg/mL).
As compared to other radical assays, the DPPH test does not require the creation of a radical molecule and is widely recognized as a reliable technique for estimating the scavenging ability of antioxidants. Electrons are paired off when DPPH radicals contact the right reducing agents, and the color of the solution declines stoichiometrically with the number of electrons absorbed. The ability of algal extracts to scavenge free radicals has frequently been investigated using this type of reactivity. The antioxidant activity of Caulerpa species was shown to be correlated with the amount of total phenolic and flavonoid contents in those species. Several studies have found that phenolic compounds are among the most effective antioxidants found in marine algae, and further research has shown that the overall phenolic content and high antioxidant activity are strongly correlated (Zakaria et al., 2011).
The emulsifying capacity of C. scalpelliformis might be due to some components which act as efficient natural emulsifiers due to the proteins that absorb at the oil–water interface by creating a steric barrier against coalescence. Polysaccharides such as alginates, agar, and carrageenan can enhance emulsion stability by raising the viscosity of the continuous phase (Bajad et al., 2024). Additionally, it was noted that seaweed polysaccharides are used commercially in a wide range of goods, including thickeners, emulsifiers, and stabilizers (Bixler & Porse, 2011).
All the detected components identified in the sulfated polysaccharides of C. scalpelliformis in this study are recognized as bioactive substances with significant therapeutic values, as demonstrated by several researchers via in vitro and in vivo investigations. For example, phenols have antimicrobial, anti-inflammatory, and antioxidant effects. As naturally occurring antioxidants, these substances have significant qualities such as stabilization of ascorbic acid, directly constricting capillaries, inhibiting lipid peroxidation, and naturally occurring phytohormones (Tanase et al., 2014). Dodecanoic acid and methylester compounds showed antioxidant, antibacterial, antiviral, and hypocholestrolemic activities. Odecanoic acid and 1,2,3-propanetriyl ester are known to have hypercholesterolemic, antiarthritic, nematocidal, and hepatoprotective activities (Omotosho et al., 2014). The other esters and derivatives of n-dodecanoic acid have also been reported for their antimicrobial, antioxidant, and anticancer activities, and 9-octadecanoic acids have antimicrobial activity (Ueda et al., 2002). These research findings clearly depict that the phytochemical compounds identified in polysaccharides of C. scalpelliformis have their own pharmacological effects and biological significance.
5. CONCLUSION
The findings of the present study clearly depict that the phytochemical compounds identified in C. scalpelliformis have their own biological significance. C. scalpelliformis can be used as a therapeutic agent for treating various ailments and for other pharmacognostic activities. Further studies are needed to evaluate their mechanism of biological efficacy and other biological and pharmacological activities.
ETHICAL CONSIDERATIONS
Not applicable (the authors are committed to assuming full responsibility in this regard).
AUTHORS CONTRIBUTIONS
Collection and/or assembly of data, Data analysis and interpretation, Writing and critical revision of the article: V.S., Data analysis and interpretation, Writing of the article: S.V., S.S., Research concept and design, Data analysis and interpretation: Si.S., Critical revision and final approval of the article: S.S.