1. INTRODUCTION
According to United Nations Children's Fund (UNICEF, 2018) data, only 41% of infants aged <6 months receive exclusive breastfeeding. The onset of mastitis is a significant factor contributing to the cessation of breastfeeding among mothers (Schwartz et al., 2002; Wöckel et al., 2008). Approximately 3–20% women experience lactational mastitis, particularly within the first 6–8 weeks postpartum (Pevzner & Dahan, 2020). Mastitis typically develops due to increased pressure within the mammary glands, resulting from increased glandular tissue and milk ducts, heightened cellular activity, and an elevated volume of milk (Wöckel et al., 2008). This condition leads to milk stasis, raises intraductal pressure, and compromises intercellular junctions within mammary epithelial cells. Consequently, increased pressure and permeability result in milk leakage into connective tissue, generating inflammation that may become complicated by secondary bacterial infections (Wöckel et al., 2008). Histopathological examinations of mammary glands in lipopolysaccharide (LPS)-induced mastitis models in mice revealed inflammatory cell infiltration and structural disruptions within acinar structures and lumina. (Eslami et al., 2015)
During episodes of mammary gland inflammation, milk stasis can lead to histological changes characterized by an increase in various cell types, such as neutrophils, eosinophils, lymphocytes, macrophages, and tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6) as inflammatory cytokines (Eslami et al., 2015; Liu et al., 2020; Tuaillon et al., 2017). High concentrations of TNF-α induce oxidative stress within tissues, resulting in vasodilation, edema, and leukocyte adhesion to epithelial surfaces, indirectly contributing to fever during inflammation (Page et al., 2018). Interleukin-1 (IL-1) serves as a key regulator of inflammation, modulating innate immune processes; elevated levels of IL-1β promote neutrophil activation and T helper 17 (Th17) cells-dominant immunopathology while facilitating monocyte differentiation into conventional dendritic cells and M1-like macrophages (Kaneko et al., 2019). Additionally, IL-6 is expressed in cellular stress conditions, such as inflammation and infection, and is involved in acute phase response as examined in the increase of acute-phase proteins, such as C-reactive protein (CRP) (Page et al., 2018).
These cytokines activate reactive oxygen species (ROS) that has a crucial contribution in inflammatory process. ROS causes oxidative damage to mammary gland tissue, adversely affecting milk secretion (Li et al., 2015). The accumulation of ROS interrupts cellular homeostasis and results in oxidative stress and mitochondrial dysfunction. Increased levels of ROS can be quantified by measuring malondialdehyde (MDA) levels (Tirani & Haghjou, 2019). Excessive ROS may induce cell death through both extrinsic and intrinsic pathways. The following four primary cell death receptors mediate the extrinsic pathway: TNF-related apoptosis-inducing ligand receptor 1 (TRAIL-R1), TRAIL receptor 2 (TRAIL-R2), TNF receptor 1 (TNFR1), and fetal alcohol syndrome (FAS or CD95) receptor (Sordillo, 2005). The binding of TNFα, FAS ligand, and TRAIL to these receptors triggers inflammatory processes. (He et al., 2017)
Antioxidant levels are essential in incidence, duration, and severity of mastitis. Elevation of serum retinol is linked to a reduced risk of developing clinical mastitis during early lactation (Leblanc et al., 2004). Low plasma levels of vitamin A and beta-carotene are associated with increased severity of mastitis in cows (Ellah, 2013).
Beta-carotene is the most prevalent carotenoid form and serves as a provitamin A compound found in high concentrations in plasma (Crackers & Per, 2019; Lesmana et al., 2020). Beyond its role as a vitamin A precursor, beta-carotene exhibits antioxidant and anti-inflammatory properties (Cheng et al., 2021; Palozza, 2005). It stimulates repair mechanisms for damaged cellular structures, activates antioxidant defenses, and inhibits pro-inflammatory responses critical for cell survival (Lesmana et al., 2020; Yang et al., 2021). Furthermore, beta-carotene suppresses intracellular ROS production while reducing MDA levels and enhancing superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity (Zhao et al., 2020). Despite these beneficial properties, limited research has explored beta-carotene's specific potential as a regulator of pro-inflammatory cytokines within the context of mastitis. This study evaluated the effects of beta-carotene supplementation during pregnancy on serum concentrations of TNF-α, IL-6, IL-1β, and MDA as well as on the histological structure of the mammary gland in a rat model of LPS-induced postpartum mastitis.
2. MATERIAL AND METHODS
2.1. Materials
The beta-carotene used in this study (catalog No. C9750-10G; Sigma-Aldrich, St. Louis, MO, USA) was a synthetic compound with a purity of ≥93% and a molecular potency of approximately 1,600,000 units of vitamin A activity per gram. LPS (catalog No. L2637; Sigma-Aldrich), derived from Escherichia coli serotype O55:B5, was purified via gel filtration. Malondialdehyde (MDA) levels were measured using the lipid peroxidation (MDA) assay kit (catalog No. MAK085; Sigma-Aldrich). Immunoassay tests for TNF-α, IL-6, and IL-1β were performed using the MILLIPLEX® rat cytokine/chemokine magnetic bead panel (catalog No. RECYTMAG-65K; Sigma-Aldrich). All reagents were supplied by PT Elo Karsa Utama (Jakarta, Indonesia).
2.2. Animals and experimental groups
This study utilized 24 healthy pregnant female Wistar mice obtained from the Laboratory Animal Center at the Faculty of Medicine, Padjadjaran University (Bandung, Indonesia). The mice were aged 8–12 weeks and weighed 200–250 g, all in their first pregnancy. Mice were kept in standard plastic cages under controlled conditions with a 12-hour light–dark cycle, an ambient temperature of 23 ± 2°C, and a relative humidity of 55 ± 5%. They had ad libitum access to a basal diet and distilled water. Subsequently, the animals were randomly distributed into the following six groups (n = 4 per group): (a) negative control group 1; (b) LPS-induced mastitis group 2; (c–e) groups 3–5 with LPS-induced mastitis receiving low, medium, and high doses of beta-carotene, respectively; and (f) LPS-induced mastitis treated with ibuprofen, 10 mg/kg body weight (bw), group 6.
After an acclimation period, two female mice were paired with one male in a single cage for 24 hours to facilitate mating (Eslami et al., 2015). Presence of a vaginal plug confirmed pregnancy, which is considered as the first day of gestation. From the gestation day 10 until 72 hours postpartum, beta-carotene groups 3–5 were administered beta-carotene doses of 20 mg/kg (low dose), 40 mg/kg (moderate dose), and 60 mg/kg (high dose) bw (Cui et al., 2012; Gu et al., 2009). Beta-carotene was mixed with sunflower oil to facilitate administration; sunflower oil was selected due to its low beta-carotene content, minimizing potential interference with results (Nikolova et al., 2012). The dosing regimen was based on previous studies demonstrating significant anti-inflammatory effects at these concentrations (Cui et al., 2012).
At 72 hours after parturition, the mice were anesthetized using sodium pentobarbital dosage of 45 mg/kg bw. The fourth pair of mammary nipples was aseptically prepared using 70% ethanol. A 1-mm section of each nipple was excised with sterile scissors to expose the lactiferous ducts. A total of 10 µg of LPS from Escherichia coli (0.1 mg/mL) dissolved in 100 µL of pyrogen-free sterile saline solution was injected into each nipple using a 32G needle, followed by a light massage to enhance the distribution of LPS (Gu et al., 2009; Kan et al., 2021). The negative control group 1 received an infusion of an equivalent volume of saline solution (100 µL). The intramammary infusion was performed according to the method described by Kai et al., 2005, in which Escherichia coli endotoxin was administered into the fourth (abdominal) mammary gland through the teat duct 72 hours after parturition (Kai et al., 2005). After 3 hours of LPS-induced mastitis, mice in group 6 received a single dose of ibuprofen (10 mg/kg bw) via intraperitoneal injection as a positive control (Tavares et al., 2006). After 24 hours of LPS induction, the mice were placed in a closed chamber with isoflurane vapor until respiratory arrest was confirmed (Leary et al., 2020). After euthanasia, mammary tissue was harvested for histopathological evaluation, and blood was collected to measure the levels of TNF-α, IL-6, IL-1β, and MDA. The blood samples were then centrifuged at 3000 rpm at 4°C for 10 minutes to isolate serum (Cui et al., 2012).
All procedures involving animals in this study were reviewed and approved by the Research Ethics Committee of the Ministry of Education, Culture, Research, and Technology at Universitas Padjadjaran (Approval No.: 1429/UN6.KEP/EC/2023).
2.3. Analysis of serum TNF-α, IL-6, and IL-1β
Serum levels of TNF-α, IL-6, and IL-1β were quantified using a multiplex immunoassay employing Luminex® technology. The MILLIPLEX® rat cytokine/chemokine magnetic bead panel assay involved adding diluted serum samples to wells containing magnetic beads pre-coated with antibodies specific to TNF-α, IL-6, and IL-1β. The blood samples were centrifuged at 1000×g for 10 minutes, after which they were diluted in assay buffer. Following the addition of samples and incubation at room temperature for 2 hours, wells were washed before detection antibodies were introduced along with streptavidin–phycoerythrin conjugate to generate a fluorescent signal. Fluorescence intensity (expressed as pg/mL) corresponding to analyte concentrations was measured using a Luminex® instrument.
2.4. Analysis of serum MDA
The MDA assay was performed according to the manufacturer’s standard protocol. Initially, 20 µL of serum or plasma was combined with 500 µL of 42-mM sulfuric acid and 125 µL of phosphotungstic acid solution; these were vortexed and allowed to incubate at room temperature for 5 minutes. Subsequently, the samples were centrifuged at 13,000×g for 3 minutes, after which the supernatant was discarded and the pellet was resuspended in 200 µL of water–butylated hydroxytoluene (BHT) solution. Finally, to form the MDA–thiobarbituric acid (TBA) adduct, 600 µL of TBA solution was added to each sample, followed by incubation at 95°C for 60 minutes and cooling in an ice bath. The reaction mixture was subsequently mixed with 1-butanol and NaCl before centrifugation; the upper butanol layer was removed for absorbance measurement at 532 nm, with the results expressed as nmol/mL.
2.5. Assessment of histopathological changes
Mammary tissue samples were immersed in 10% neutral buffered formalin for 24 hours before processing into tissue blocks. Subsequent steps included alcohol dehydration, clearing with xylene, impregnation with paraffin wax, and embedding into blocks, followed by sectioning into 5-µm-thick slices. The paraffin sections underwent deparaffinization with xylene and hydration through a series of graded alcohol concentrations (110, 100, 95, 90, 80, and 70%). Staining was performed using hematoxylin and eosin (H&E), followed by mounting with entellan (Eslami et al., 2015; Kan et al., 2021). Histological evaluations were conducted using a 200× magnification microscope and scored based on the following criteria (Gogoi-Tiwari et al., 2017):
2.6. Data Analysis
Statistical analyses were conducted using SPSS version 22.0. Initially, data normality was assessed using the Shapiro–Wilk test; customarily distributed data (p > 0.05) proceeded to ANOVA analysis followed by Tukey’s least significant difference (LSD) test for multiple comparisons if significant differences were identified. Welch’s ANOVA was employed to analyze non-homogeneous variances among the groups, and the Games–Howell test was used for post hoc analysis.
3. RESULTS
3.1. Body Weight of Experimental Animals
3.1.1. Maternal Body Weight
Maternal body weight was measured on the termination day of the study to evaluate whether the treatments induced significant differences in experimental groups. A series of statistical analyses was conducted, beginning with normality and homogeneity tests. The results of the normality test indicated that all groups followed a normal distribution as all p-values exceeded the significance threshold of α = 0.05. Specifically, the p-values were as follows: negative control, p = 0.687; LPS-induced without treatment, p = 0.781; low-dose beta-carotene, p = 0.865; moderate-dose beta-carotene, p = 0.418; high-dose beta-carotene, p = 0.537, and LPS induced with ibuprofen, p = 0.121. The homogeneity test further confirmed equal variance among groups, yielding p = 0.414.
Subsequently, a one-way ANOVA was performed to compare maternal body weight across the groups. The analysis did not detect any statistically significant differences in maternal body weight in all the groups (F = 0.068, p = 0.996). The mean maternal body weight (±SEM) for each group was as follows: negative control, 278 ± 11.65 g; LPS induced without treatment, 280.75 ± 23.10 g; low-dose beta-carotene, 270.75 ± 9.19 g; moderate-dose beta-carotene, 277.25 ± 8.37 g; high-dose beta-carotene, 269.75 ± 21.35 g; and LPS induced with ibuprofen, 276.75 ± 19.53 g. These findings suggest that the treatments, including beta-carotene at various doses and ibuprofen, did not significantly impact maternal body weight (Table 1).
Table 1
One-way ANOVA analysis of the effects of beta-carotene administration at three different doses on maternal and offspring body weight.
3.1.2. Offspring Body Weight
The body weight of offspring from all experimental groups was measured on the termination day to evaluate potential differences attributable to all treatments. As with maternal body weight, the data underwent normality and homogeneity testing. The results of the normality test demonstrated that all groups followed a normal distribution, with p-values exceeding the significance threshold of α = 0.05. Specifically, the p-values were as follows: negative control, p = 0.824; LPS induced without treatment, p = 0.919; low-dose beta-carotene, p = 0.845; moderate-dose beta-carotene, p = 0.884; high-dose beta-carotene, p = 0.944; and LPS induced with ibuprofen, p = 0.941.
Additionally, the homogeneity test confirmed that variance across all groups was equal, yielding a p = 0.515. The one-way ANOVA analysis for offspring body weight revealed no significant differences among all groups (F = 1.646, p = 0.199). The mean offspring body weight (±SEM) for each group was as follows: negative control, 8.15 ± 0.70 g; LPS induced without treatment, 8.42 ± 0.82 g; low-dose beta-carotene, 8.14 ± 0.22 g; moderate-dose beta-carotene 8.03 ± 0.47 g; high-dose beta-carotene, 10.00 ± 0.83 g; and LPS induced with ibuprofen, 9.20 ± 0.37 g. These results indicate that the treatments did not significantly alter offspring body weight.
Overall, these findings demonstrated that beta-carotene supplementation at various doses and ibuprofen treatment did not significantly affect the body weight of either maternal or offspring animals, suggesting that these interventions were well tolerated in terms of weight stability in this experimental model.
3.2. Regulation of Inflammatory Cytokine Responses
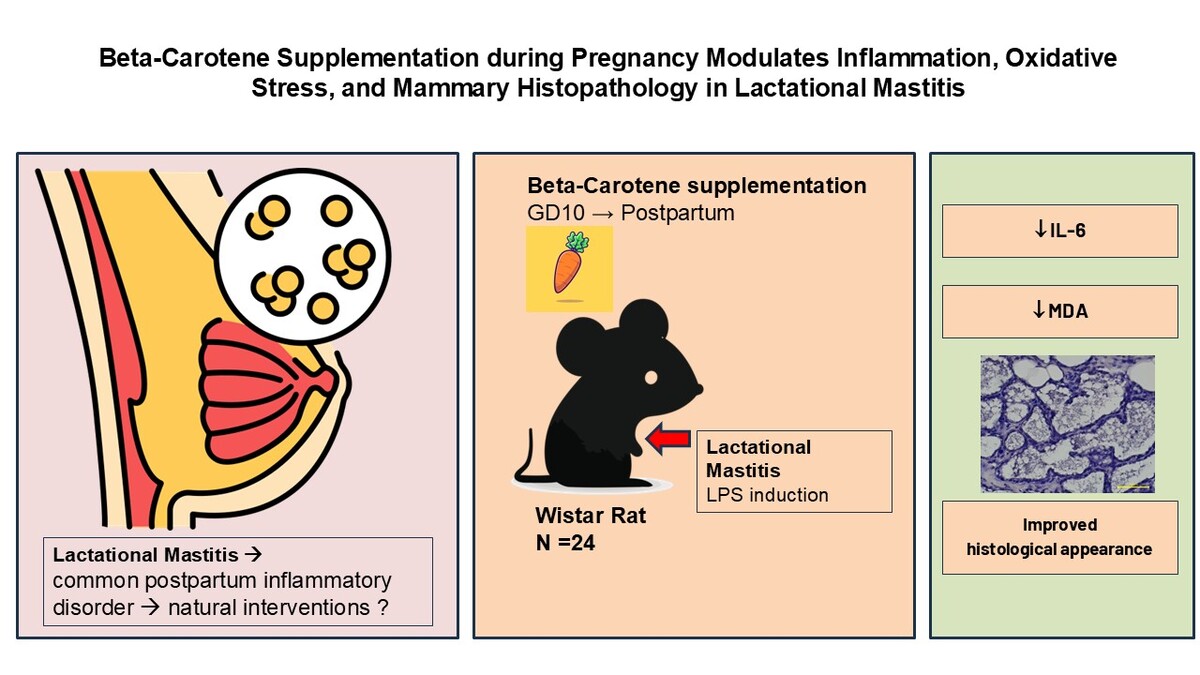
A normality test confirmed that all experimental groups exhibited a normal distribution of serum TNF-α levels, with p-values exceeding the significance threshold of α = 0.05. The homogeneity test indicated equal variance among groups, allowing for the use of one-way ANOVA. The one-way ANOVA did not reveal significant differences in TNF-α levels across treatment groups (F = 2.199, p = 0.100), and no further analysis was conducted. Mean TNF-α levels (±SEM) were as follows: negative control, 2.11 ± 1.03 pg/mL; LPS induced without treatment, 8.25 ± 3.36 pg/mL; low-dose beta-carotene, 4.19 ± 1.00 pg/mL; moderate-dose beta-carotene, 3.23 ± 0.69 pg/mL; high-dose beta-carotene, 2.50 ± 0.80 pg/mL; and LPS induced with ibuprofen, 2.26 ± 0.59 pg/mL. These results presented that supplementation with beta-carotene during pregnancy has the tendency to lower serum TNF-α levels in lactating female Wistar mice subjected to LPS-induced mastitis, indicating its potential as an anti-inflammatory agent in this model.
The one-way ANOVA results revealed significant differences in serum IL-6 levels among treatment groups (F = 3.219, p = 0.030). The mean serum IL-6 levels (±SEM) were as follows: negative control, 200.62 ± 37.94 pg/mL; LPS induced without treatment, 429.12 ± 108.90 pg/mL; low-dose beta-carotene, 255.10 ± 45.46 pg/mL; moderate-dose beta-carotene, 170.89 ± 60.02 pg/mL; high-dose beta-carotene, 142.94 ± 54.61 pg/mL; and LPS induced with Ibuprofen, 104.16 ± 55.32 pg/mL. Notably, the LPS-induced group without treatment exhibited elevated IL-6 levels, compared to the negative control group, while beta-carotene administration resulted in decreased IL-6 levels, particularly at a high dose. Post hoc LSD analysis further confirmed significant differences between the LPS-induced group and the negative control group (p = 0.022) as well as between the LPS-induced group and those receiving moderate (p = 0.011) and high (p = 0.006) doses of beta-carotene, indicating that beta-carotene effectively reduces IL-6 levels in this model of mastitis.
The results indicated a trend toward increased serum IL-1β levels in the LPS-induced group without treatment (60.42 ± 8.82 pg/mL), compared to the negative control group (46.61 ± 10.33 pg/mL). In contrast, administration of beta-carotene resulted in a notable decrease in IL-1β levels, particularly at the moderate dose (26.66 ± 6.07 pg/mL), followed by the high dose (52.76 ± 6.81 pg/mL). The one-way ANOVA results were not statistically significant, with p = 0.054, but the findings suggest a potential trend aligned with theoretical expectations regarding the anti-inflammatory effects of beta-carotene. Figure 1 presents graphical data illustrating the effects of beta-carotene supplementation on cytokine responses.
Figure 1
Impact of beta-carotene on inflammatory cytokine responses, oxidative stress mechanisms, and mammary gland inflammation is illustrated by the measurements of (A) TNF-α, (B) IL-6, (C) IL-1β, (D) MDA, and (E) histological score. Data are presented as mean ± SEM for four mice; *p < 0.05 is statistically significant.
3.3. Comparative Effects of Three Doses of Beta-Carotene on Serum MDA Levels in Lactating Female Wistar Mice Induced with LPS
Following the homogeneity test, which yielded p = 0.007, indicating non-homogeneous variances among the groups, Welch’s ANOVA was employed to analyze the impact of beta-carotene supplementation at three different doses on serum MDA levels in LPS-induced mastitis in lactating female Wistar mice. The results demonstrated significant differences in serum MDA levels across all treatment groups (F = 11.700, p = 0.005). Specifically, the mean serum MDA levels were significantly elevated in the LPS-induced group without treatment (73.11 ± 20.68 pg/mL), compared to the negative control group (7.79 ± 2.80 pg/mL). Notably, beta-carotene administration resulted in a marked reduction in MDA levels, with the lowest concentration observed in the moderate-dose beta-carotene group (7.15 ± 3.47 pg/mL), followed by the high-dose beta-carotene group (13.19 ± 4.91 pg/mL).
Post hoc analysis using the Games–Howell test revealed significant differences between the LPS-induced group and all treatment groups, including the negative control (p < 0.001), low-dose beta-carotene (p < 0.001), moderate-dose beta-carotene (p < 0.001), high-dose beta-carotene (p < 0.001), and Ibuprofen treatment (p < 0.001). These findings indicate that these results aligned with theoretical expectations regarding the anti-inflammatory properties of beta-carotene, suggesting its potential efficacy in reducing oxidative stress as measured by serum MDA levels in this experimental mastitis model. Figure 1 presents the impact of beta-carotene supplementation on serum MDA levels.
3.4. Alterations in Mammary Gland Histopathology
The analysis of histopathological inflammation scores in the mammary tissues of lactating female Wistar mice induced with LPS revealed significant differences among the treatment groups. There was a substantial increase in the average histological score for the LPS-induced group without treatment (2.14 ± 0.14), compared to the negative control group (0.19 ± 0.08), demonstrating the inflammatory response induced by LPS. Administration of beta-carotene resulted in a notable reduction in inflammation scores, with the lowest score observed in the high-dose beta-carotene group (1.09 ± 0.44), followed closely by the moderate-dose beta-carotene group (1.10 ± 0.29). The overall ANOVA analysis yielded p < 0.001, indicating a statistically significant effect of beta-carotene supplementation on histopathological scores.
Post hoc analysis using LSD tests further confirmed meaningful differences between the LPS-induced group and the negative control group (p = 0.000) as well as between the LPS-induced group and both moderate-dose (p = 0.013) and high-dose beta-carotene groups (p = 0.012). Additionally, significant differences were noted between the low-dose beta-carotene group and the negative control group (p = 0.000) as well as between the low-dose beta-carotene and both moderate-dose beta-carotene group (p = 0.005) and high-dose beta-carotene group (p = 0.004). These findings suggest that while inflammation scores were elevated due to LPS induction, beta-carotene supplementation effectively mitigated these inflammatory responses, supporting its potential therapeutic role in managing mastitis.
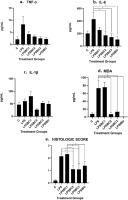
To assess the impact of beta-carotene supplementation on inflammation in mammary tissue, histological evaluations were conducted across various treatment groups. Figure 2 presents representative histopathology features from each group, demonstrating distinct inflammatory responses. The LPS-induced group without treatment exhibited significant infiltration of inflammatory cells, as shown in panel B, compared to the negative control group, depicted in panel A. Notably, panels D and E illustrate reduced inflammation scores in both moderate-dose and high-dose beta-carotene groups, indicating a potential therapeutic effect of beta-carotene.
Figure 2
Histopathological changes in the mammary tissues of Wistar mice with LPS-induced mastitis under different treatments. A: Control group: mammary tissue structure with intact alveoli, no inflammatory infiltration, and preserved glandular architecture (score 0). B: LPS induced without treatment: severe inflammatory changes with extensive cellular infiltration, tissue necrosis, alveolar damage, and disruption of glandular structure (score 3). C: Low-dose beta-carotene (20 mg/kg BW): moderate inflammatory cell infiltration with partial preservation of alveolar structures and reduced necrosis, indicating a protective effect of pre-administration of beta-carotene, compared to the LPS-induced group (score 2). D: Moderate-dose beta-carotene (40 mg/kg BW): mild inflammatory changes with improved alveolar structure, reduced cellular infiltration, and minimal tissue damage, suggesting a dose-dependent protective effect of beta-carotene pre-administration (score 1). E: High-dose β-carotene (60 mg/kg BW): mammary tissue structure with intact alveoli, no inflammatory infiltration, and preserved glandular architecture (score 0). F: LPS induced with ibuprofen treatment: mammary tissue structure with intact alveoli, no inflammatory infiltration, and preserved glandular architecture (score 0). Hematoxylin and eosin (H&E) staining was performed on mammary tissue sections. Panels A1–F1 depict images captured at ×100 magnification, while panels A2–F2 present images at ×400 magnification.
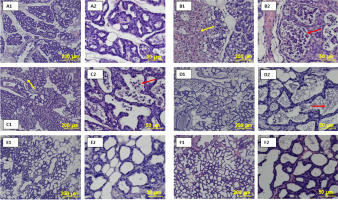
Figure 3
Mechanism of beta-carotene in attenuating LPS-induced mastitis. LPS activates the TLR4/MyD88 signaling cascade, leading to the activation of NF-κB and MAPK (ERK, JNK, p38), increased ROS, MDA, and proinflammatory cytokines (TNF-α, IL-6, IL-1β), resulting in tissue damage. Beta-carotene inhibits ROS accumulation and suppresses NF-κB and MAPK activation, thereby reducing cytokine release and oxidative stress, and improving mammary gland histology. Carrot icons indicate points of beta-carotene action.
Figure 2 presents histopathological analysis of mammary tissues from Wistar mice, demonstrating significant variations among treatment groups. In the (a) negative control group, the mammary gland exhibits normal architecture with intact alveoli and minimal inflammatory infiltration. In contrast, the (b) LPS-induced group without treatment shows extensive inflammatory changes, including severe cellular infiltration, widespread necrosis, and disrupted alveolar structures, indicating severe mastitis. Red arrows in the figure show neutrophil infiltration and yellow arrows show structural damage of mammary glands. Treatment with (c) low-dose beta-carotene results in moderate improvement, characterized by reduced inflammation and partial preservation of alveolar structures. Notably, the (d) moderate-dose beta-carotene group exhibits further improvement, with mild inflammatory changes, better alveolar integrity, and minimal tissue damage. The most significant recovery is observed in the (e) high-dose beta-carotene group, where the mammary tissue appears nearly normal, with minimal inflammatory infiltration, restored glandular architecture, and reduced necrotic areas. These findings suggest that beta-carotene, particularly at higher doses, effectively alleviates histopathological damage caused by LPS-induced mastitis, comparable to the standard anti-inflammatory treatment with ibuprofen.
4. DISCUSSION
Lactational mastitis is a prevalent inflammatory breast condition that typically arises during breast feeding, especially in the initial postpartum weeks. Preventive measures utilizing natural and readily accessible substances are particularly valuable, especially those that provide additional health benefits for both mother and developing fetus. Vitamin A is indispensable for the fetus’s and newborn’s proper growth and development. Supplementing with vitamin A during pregnancy is pivotal for maintaining maternal health and ensuring optimal fetal developmental outcomes. It enhances maternal immune function (Nishiyama et al., 2021), reduces the risk of pregnancy-related complications (Oliveira-Menegozzo et al., 2010), and supports the growth and differentiation of fetal tissues. Adequate vitamin A levels are essential for fetal organ development, particularly the lungs, heart, and eyes, and contribute to improved neonatal immunity through the transfer of immunoglobulins via breast milk (Nishiyama et al., 2021). Beta-carotene is a precursor to vitamin A, allowing the body to convert it into active vitamin A (retinol) as needed. This minimizes the risk of hypervitaminosis A, a condition associated with excessive vitamin A intake. Unlike preformed vitamin A, such as retinol or retinyl esters, beta-carotene is nontoxic even at high doses, as the conversion to retinol is tightly regulated by the body, reducing the likelihood of toxicity (Guo et al., 2019a). In addition to serving as a vitamin A source, beta-carotene acts as a potent antioxidant, protecting cells from oxidative stress and reducing inflammation, which are critical during pregnancy (Oliveira-Menegozzo et al., 2010). Given its well-established anti-inflammatory and antioxidant properties, along with its documented benefits for maternal and fetal health, beta-carotene presents a promising candidate for inclusion in prenatal supplementation regimens.
Bacterial infections frequently precipitate mastitis, triggering a series of immune responses that engage both innate and adaptive mechanisms (Wang et al., 2021; Wilson et al., 2020). At the outset, pathogen-associated molecular patterns (PAMPs), such as LPS from Gram-negative bacteria and peptidoglycans from Gram-positive bacteria are detected by pattern recognition receptors, notably toll-like receptors (TLRs). In particular, TLR4 is critical for recognizing LPS, whereas TLR2 is responsible for identifying components from Gram-positive bacteria (Zou et al., 2020). The activation of these receptors triggers signaling pathways, including nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK), which lead to the transcription of pro-inflammatory cytokines. For instance, TNF-α is rapidly produced, increasing vascular permeability and promoting the recruitment of neutrophils and monocytes. Additionally, IL-1β intensifies inflammatory response by triggering inflammasome activation whereas IL-6 supports acute-phase reaction by inducing acute-phase protein synthesis and further attracting immune cells (Gogoi-Tiwari et al., 2017).
To study the possible efficacy of beta-carotene for preventing mastitis, we established in vivo rat models using LPS as an inflammation inducer. LPS was used to induce mastitis because it is a potent endotoxin derived from Gram-negative bacterial cell walls, widely recognized for eliciting an acute inflammatory response in mammary tissues (Eslami et al., 2015; Guo et al., 2019b; Kan et al., 2021). LPS administration effectively simulates clinical and subclinical mastitis without introducing the complexity and variability of live bacterial infections, enabling controlled and reproducible assessment of inflammatory responses, oxidative stress mechanisms, and tissue pathology. Key endpoints, including body weight stability, inflammatory cytokine levels (TNF-α, IL-6, and IL-1β), serum MDA, and mammary gland histopathology, were evaluated to determine the potential of beta-carotene in preventing lactational mastitis. The results provide substantial evidence supporting its beneficial role in mitigating inflammation and oxidative stress, particularly at moderate and high doses. Wistar mice were selected for this study because they represent a well-established animal model widely accepted for investigating inflammatory responses, oxidative stress, and mammary gland pathology (Akkara & Sabina, 2020b; Cui et al., 2012; Dewi et al., 2020) Using Wistar mice ensures comparability of results with existing literature and facilitates reproducibility of experimental outcomes.
The body weight of maternal mice and their offspring was not significantly affected by beta-carotene supplementation or ibuprofen treatment, as indicated by nonsignificant ANOVA results (p > 0.05). These findings suggest that the interventions did not adversely impact general health, weight stability, or the overall development. The consistent body weight across treatment groups highlights the safety and tolerability of beta-carotene in this experimental model. This result aligns with the findings of Yang et al. (2021), who reported no significant differences in body weight among four groups at baseline and after 15 days of beta-carotene supplementation.
Beta-carotene supplementation during pregnancy produced dose-dependent decrease in pro-inflammatory cytokine levels, with the most substantial reduction occurring at moderate and high doses. In an LPS-induced mastitis model, the inflammatory profile was marked by significantly elevated serum IL-6 and a tendency for increased TNF-α and IL-1β concentrations. Although the one-way ANOVA for TNF-α did not demonstrate statistical significance (p = 0.100), the observed trend indicates a clinically meaningful reduction in TNF-α levels with beta-carotene treatment. Notably, the high-dose group exhibited TNF-α levels (2.50 ± 1.60 pg/mL) similar to those in the ibuprofen-treated group, underscoring beta-carotene’s potential anti-inflammatory efficacy. These findings are consistent with previous studies demonstrating beta-carotene’s role in modulating inflammatory cytokine production through its antioxidant mechanisms (Akkara & Sabina, 2020a; Kawata et al., 2018).
A statistically significant decrease in IL-6 levels was observed (p = 0.030), particularly in the moderate- and high-dose beta-carotene groups. Post hoc LSD analysis confirmed meaningful differences between the LPS-induced group and the moderate-dose (p = 0.011) and high-dose (p = 0.006) beta-carotene groups. IL-6 is a key mediator in acute inflammatory response, and its suppression by beta-carotene suggests a protective effect against LPS-induced mastitis. Although IL-1β levels notably decreased with beta-carotene supplementation, particularly at moderate doses, the one-way ANOVA results did not reach statistical significance (p = 0.054). Nonetheless, the observed trend aligns with the anti-inflammatory properties of beta-carotene and its ability to modulate IL-1β production in inflammatory conditions.
The differential effects of beta-carotene on reducing inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, may be attributed to several potential mechanisms. IL-6 production is often regulated differently from TNF-α and IL-1β during inflammation. IL-6 is primarily induced via the Janus kinase 2–signal transducer and activator of transcription 3 (JAK2/STAT3) pathway whereas TNF-α and IL-1β are more tightly controlled by the NF-κB and inflammasome pathways. Beta-carotene might specifically inhibit the pathways associated with IL-6 production (e.g., JAK2/STAT3) while having less impact on NF-κB or inflammasome activation, which drive TNF-α and IL-1β expression (Guoxia et al., 2022). IL-6 also has a role as prognostic value in early inflammation (Kany et al., 2019).
Malondialdehyde is a highly reactive aldehyde formed from lipid peroxidation, a process in which ROS break down polyunsaturated fatty acids in cell membranes (Cordiano et al., 2023). The subsequent build up of biomolecules modified by MDA can activate inflammatory pathways by stimulating immune cells and prompting the release of pro-inflammatory cytokines. Furthermore, sustained elevations in MDA levels are documented across numerous inflammatory disorders, including allergic airway conditions, such as asthma and rhinitis, highlighting its utility as a biomarker for oxidative stress and inflammation. Furthermore, MDA is capable of forming adducts with deoxyribonucleic acid (DNA), which may lead to mutagenesis and contribute to the development of cancer. Collectively, these mechanisms underscore the significant role of MDA in mediating and exacerbating inflammation associated with oxidative stress (Raghavan et al., 2012).
Serum MDA levels were significantly elevated in the LPS-induced group without treatment, indicating heightened oxidative stress associated with mastitis. Supplementation with beta-carotene markedly reduced MDA levels, particularly in the moderate- and high-dose groups. Welch's ANOVA results (p = 0.005) and subsequent post hoc analysis using Games–Howell confirmed significant differences between the LPS-induced group and all treatment groups.
Recent studies have explored the impact of beta-carotene on MDA levels, a marker of oxidative stress. In a study involving Korean adolescents, plasma beta-carotene concentrations were inversely correlated with MDA levels, particularly among males, suggesting that higher beta-carotene status may mitigate lipid peroxidation (Joo et al., 2022).
In a separate study, the effects of beta-carotene supplementation on oxidative stress biomarkers were evaluated in healthy subjects. Over a 5-week period, participants received daily doses ranging from 5 mg to 40 mg, with only the 40-mg group demonstrating a statistically significant reduction in plasma MDA levels. This finding suggests that higher doses of beta-carotene may be necessary to effectively attenuate lipid peroxidation. Beta-carotene exerts its antioxidative action by neutralizing ROS, thereby reducing the formation of MDA—a well-established marker of lipid peroxidation. Its conjugated double-bond structure facilitates the quenching of singlet oxygen and the scavenging of free radicals, thus effectively inhibiting the initiation of lipid peroxidation chains (Bohn, 2019).
Reduced MDA levels highlights beta-carotene’s efficacy in combating lipid peroxidation, a key feature of oxidative stress during inflammation. The observed improvements align with previous reports suggesting that beta-carotene is a potent antioxidant, effectively neutralizing free radicals and reducing oxidative damage to cellular membranes. Notably, the moderate-dose group achieved the lowest MDA levels (7.15 ± 6.94 pg/mL), underscoring its optimal therapeutic potential in this model.
Lipopolysaccharide-induced mastitis is distinguished by marked histopathological modifications in mammary tissue, including the infiltration of inflammatory cells, edema, and hyperemia. These alterations disrupt the integrity of blood–milk barrier, largely because of the impairment of tight junction proteins, such as claudin-3 and occludin, which further amplifies inflammatory response (Wang et al., 2017). Additionally, beta-carotene significantly contributes to tissue repair and wound-healing. Following its conversion to retinol, beta-carotene modulates a variety of biological processes, notably cellular differentiation and immune function. In the context of wound-healing, vitamin A accelerates tissue repair by promoting epidermal turnover, enhancing re-epithelialization, and restoring epithelial architecture (Polcz & Barbul, 2019).
Histological analysis revealed significant inflammatory changes in the mammary tissue of LPS-induced mice, characterized by extensive cellular infiltration, necrosis, and disrupted alveolar structures. Treatment with beta-carotene resulted in dose-dependent improvements in mammary gland histopathology. Both moderate-dose and high-dose beta-carotene groups exhibited the most notable recovery, with reduced inflammation scores and preservation of alveolar integrity. The high-dose group exhibited near-normal histological architecture, characterized by minimal inflammatory infiltration and a restored glandular structure, comparable to that of the ibuprofen-treated group. Analysis of histopathological inflammation scores revealed significant intergroup differences. Beta-carotene administration notably reduced inflammation scores, with the high-dose group showing the most pronounced decrease.
The observed reductions in cytokine levels and oxidative stress parameters, along with the preservation of mammary gland structure during mastitis, support the critical anti-inflammatory role of beta-carotene. Beta-carotene prevents inflammation by inhibiting the oxidative stress-induced inflammatory signaling pathways and tissue injury. Structurally, beta-carotene consists of a 40-carbon backbone with conjugated double bonds, which are essential for its chemical and biological activities. These conjugated double bonds are responsible for beta-carotene’s capacity to absorb light energy and significantly enhance its antioxidant properties. Specifically, beta-carotene acts as an effective radical quencher, scavenging peroxyl radicals and disrupting the chain reactions that lead to cellular damage (Kang & Kim, 2017). Beta-carotene is reported to suppress oxidative stress-induced activation of NF-κB and STAT3, thereby effectively preventing and reducing inflammation. Beta-carotene also inhibits the JAK2/STAT3, NF-κB, and c-Jun N-terminal kinase (JNK)/p38 MAPK signaling pathways, thereby reducing the expression of pro-inflammatory cytokines and preventing the progression of inflammatory responses (Guoxia et al., 2022). By neutralizing oxidative radicals, beta-carotene prevents the oxidative modification of cellular components, limiting tissue injury and inflammation progression. Beta-carotene also modulates immune function by influencing the differentiation, activation, and functioning of immune cells, including macrophages and T-cells (Ross, 2012). This contributes to the resolution and prevention of excessive or chronic inflammatory responses.
The findings of this study demonstrate promising anti-inflammatory and antioxidant effects of beta-carotene supplementation in a rodent model of mastitis, suggesting potential clinical applications in humans. However, direct translation to human clinical practice requires caution. Additional studies are essential, including well-designed clinical trials, to evaluate safety, efficacy, optimal dosing, and long-term outcomes of beta-carotene supplementation in pregnant or lactating women.
This study has several limitations that must be considered. First, the use of an LPS-induced mastitis model, although standardized and reproducible, does not fully capture the complexity of naturally occurring bacterial mastitis. Future studies should consider evaluating beta-carotene supplementation in models involving live bacterial infections to better mimic clinical scenarios. Second, the duration of beta-carotene supplementation and the timing of inflammatory marker assessments were relatively short-term issues; thus, long-term studies are necessary to fully understand chronic effects and potential implications during lactation. Lastly, the precise molecular mechanisms underlying beta-carotene's anti-inflammatory and antioxidant actions are not elucidated fully and warrant further investigation.
5. CONCLUSION
Beta-carotene supplementation at moderate and high doses significantly mitigates inflammation, oxidative stress, and histopathological damage in LPS-induced mastitis in lactating Wistar mice. These findings highlight the potential of beta-carotene as a safe and effective natural intervention for inflammatory conditions, such as mastitis, providing a promising alternative to conventional anti-inflammatory treatments.
AUTHORS CONTRIBUTIONS
Stella Tinia Hasianna, Julia Windi Gunadi, Enny Rohmawaty, and Ronny Lesmana were responsible for conceptualizing and designing of the study. Stella Tinia Hasianna and Ronny Lesmana performed laboratory experiments, collected the data, and assisted in its interpretation. Stella Tinia Hasianna also carried out statistical analyses and drafted manuscript’s initial version. The research was supervised by Ronny Lesmana, Julia Windi Gunadi, and Enny Rohmawaty, who reviewed the manuscript and provided critical feedback. Additionally, Ronny Lesmana and Julia Windi Gunadi had a key role in acquiring funding and resources for the project and critically revising the manuscript for intellectual content. All authors had read and approved the final manuscript. Data authentication was not applicable.