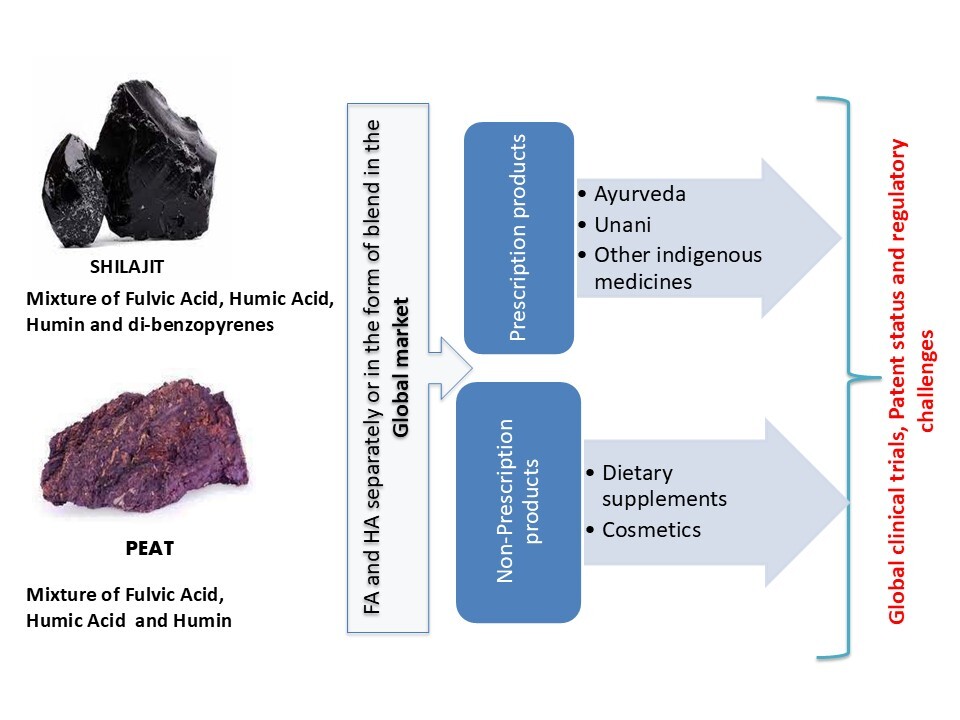
1. INTRODUCTION
Humic substances (HS) are straw-colored organic acids mainly derived from soil humus and plants. They have unique properties of exhibiting hydrophobic core and hydrophilic exterior. The major components are humic acid (HA) and fulvic acid (FA). FA is a category of HS with low molecular mass, constituting a short-chain compound that dissolves in acid or base solutions (Table 1). It comprises functional groups, such as carboxyl, phenol, hydroxyl, amine, and quinine groups, exhibiting a high complexation capacity with heavy metals (Zhong et al., 2023). HA, with a comparatively higher molecular mass, dissolves in alkaline pH and possesses several nutraceutical (NC) properties, including anti-inflammatory, antimicrobial, and immune regulation abilities.
Table 1
Physicochemical properties of fulvic acid (FA) and humic acid (HA).
[i] Note. Humic substances are complex organic macromolecules with a diverse range of properties and intricate structures; however, the physicochemical properties of humic substances are still not fully understood (Klučáková, 2016; Tarasevich et al., 2016).
Humic substances exhibit favorable impacts on plant physiology by enriching soil structure and fertility, impacting nutrient uptake and root architecture. HS are the massive constituents of soil organic matter (~60%) and are speculated to be a key ingredient of the terrestrial ecosystem. It is responsible for various complex chemical reactions that occur in the soil. One of the notable traits of HS is their capability to interact with metal ions, oxides, hydroxides, minerals, and
organic compounds, comprising toxic contaminants. The sources of HS are peat, lignite, leonardite, coal, and natural water sources, such as rivers, lakes, and streams.
Unlike other natural ingredients, the journey of HS, for example, HA and FA, as human healthcare products has been remarkable. In ancient times, HS was used as a drug in different systems of medicines that still exist in indigenous medicine streams, such as Unani (Perso-Arabic Medicine) Ayurvedic (ancient/traditional Indian system), Chinese (Zhou et al., 2014), Russian (Schepetkin et al., 2003), and other countries of the Himalayan region. With the progression of time and scientific temperament, the evidence or science-based medicine (e.g., allopathic medicine) took over, as the natural ingredient-based medicines lacked sufficient scientific evidence. Other than uses in indigenous systems of medicine, it is also being explored in other facets of healthcare. In the given scenario, HS products have following four options
To continue to be a part of traditional and alternative medicines as mentioned Tables 2, 7 and 8 (i.e., no need to generate safety and efficacy data).
To position itself in the category of dietary supplements (DS) or NC (i.e., without any therapeutic label claim)as mentioned Tables 3 and 4.
To attract the scientific community to generate scientific data and validate evidence of safety and efficacy and to be marketed as prescription products.
To be explored for other categories of healthcare products, like excipient, cosmeceuticals (Table 5), and veterinary medicines.
Figure 1
A pictorial presentation of all the available non-prescription HS-based products (dietary supplements and cosmetics/topicals). Each region is divided into three colors—blue (liquid oral HS products), green (solid oral HS products), and yellow (topical HS products). The area of any colored region is an approximate representation of percentage of that category of HS product in the particular region. For example, in Australia, almost all the HS products are of liquid oral category.
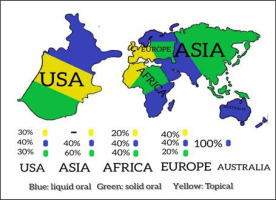
Table 2
Marketed herbal formulations containing shilajit.
Table 3
Solid oral dosage forms available in different counties.
[i] Notes. The names of the products have been given in alphanumeric form, prefixed with abbreviated country name. The data were collected from the products’ labels, websites, emails. and chatbots of various company websites. The table includes information from companies, such as HealthForce SuperFoods™, Jarrow Formulas, Physique Enhancing Science, XYMOGEN, AI Sports Nutrition, Joy to Live, Prescript Assist, Humineral, Supreme Fulvic, Biotivia, Global Healing Center, Irwin Naturals, Life Extension, MuscleTech™, Natural Dynamix, Omica Health, Patient One MediNutritionals™, Systemic Formulas Inc., Morningstar Minerals, HUmineral, Roar to Life, Natural-ly, BlackMP – Foods for Athletes, Nutricology, Mineralife, Vital humic, Isha Agro Developers Pvt. Ltd., Dabur, Health Genuine India Pvt. Ltd, Bixa Botanical, CHD-Fulvic Acid, BioBalance, Fulvic Health, Biosil, Oshun Health, Health Solution, Hymato, Life Springs, Sisel International, Zeo Natural, and Healthy 100®.
Table 4
Liquid oral products available in the global market.
[i] Notes. The names of the products are given in alphanumeric form, prefixed with abbreviated country name. The data were sourced from product labels, websites, emails, and chatbots of various company websites. The table includes information from a diverse range of companies, such as Mineralife, Supreme Fulvic, Zenful (a subsidiary of Earthwater UK), Foods for Athletes, Trace Minerals Research, Elixa, Optimally, Organic, Omica Organics™, Pure Fulvic Minerals, Mineral Logic, Alpha Health Support, Vital Earth Minerals, Phylia [de M.], ARCADIA, Drucker Labs, Mana Life Laboratory (a division of BioAg Corp), Nature’s Sunshine, Ayurvedic Rasayanas, BodyHealth, Designs for Health, Get Healthy Again, Global Healing Centre, Global Health Trax, InfoWars Life, InfoWars Life Super, Liquid Zeolite Co. Inc., Purium, Nature’s Sunshine, New Sun®, NOW®, NutraMedix, Protocol for Life Balance, Vitabase, Protect Cells, Morningstar Minerals, Trace Minerals Research, Dirobi, Doc of Detox, Vital Earth Minerals, Motherearthlabs, Pure Planet, Creative Concept Labs, Cell Cynergy Nutrition, Vital Earth Minerals, Natural Health Orgnics, Herb Anomic, Mommys Miracles, SBR Nutrition, Leaf Source, Trace Minerals, Athletic Elite 10, Sunwarrior, FulvicXcell Products Ltd., Humalife, American Nutriceuticals, Innotech Nutrition, Fulhold Pharma Ltd., Fulvic Force, CHD-Fulvic Acid, Sfera Bio Nutrition, Oshun Health, Oshun Health, Reach for Life, Life Springs, GO2 Liquid Minerals, Alkaline, Athletes Advantage, Blk Beverages, Earth water, 77 Blackwater, Ancient Purity, I.S. Natura, Hymato, Health Solution, and NuCell+.
Table 5
Topical or cosmetic products available in global market, encompassing spritz, sprays, creams, lotions, gels, body cleanser, shampoo, eye drops, eye creams, conditioners, nasal spray, throat spray, wound care spray, cleansing balm, bath powder, and face mask.
Table 6
Examples of some natural ingredients used across different categories of products.
[i] Notes. $The Inactive Ingredients Database of US FDA can be accessed at https://www.fda.gov/drugs/drug-approvals-and-databases/inactive-ingredients-database-download. *The Handbook of Pharmaceutical Excipients is a nonofficial compendium and is widely referred for information on excipients.
Table 7
Formulations from the Unani system of medicine.
| S. No. | Name of product | Ingredient (s) | Recommended dose (dosage form) | Proportion of shilajit used | Indication(s) | References |
|---|---|---|---|---|---|---|
| 1. | Sufoof-e-Suzak Qawi | Zizyphus jujube, Butea monosperma, Tinospora cordifolia, shilajit,Rheum officianale,and +6 | 5–10 g | 5% | Gonorrhoea | Yusufuddin, 2006 |
| 2. | Habb-e-Suzak Muzmin | Bergenia ligulata, Ipomoea alba, Elettaria cardamomum, Terminalia chebula,shilajit,and+3 | 3 g (tablet) | 8.5% | Gonorrhoea and burning micturition | Yusufuddin, 2006 |
| 3. | Sufoof-e-Ziabetus Qawi | Cochlospermum religiosum, Bambusa bambos, Aluminium silicate, marl, shilajit,and +17 | 3–5 g | 4% | Polyurea, diabetes insipidus, and renal insufficiency | Yusufuddin, 2006 |
| 4. | Habb-e-Luknat | Myrica esculenta, Rosa damascena, Cymbopogon citratus, Syzygium aromatic, shilajit,and +3 | 550 mg–1g (tablet) | 5.5% | Stammer | Yusufuddin, 2007 |
| 5. | Majoon-e-Regmahi | Sphenomorphus indicus, Holarrhena antidysentrica, Papaver somniferm, Curcuma zedoaria, shilajit,and +30 | 5–10 g (Oral/semi-solid) | 1% | Sexual weakness and spermatorrhoea | Yusufuddin, 2008 |
| 6. | Habb-e-Mumsik Tilai | Hyoscyamusniger, Centaureabehen,Papaver somniferm, Datura metel, shilajit,and +10 | 600 mg daily (tablet) | 4% | Sexual weakness and nervine weakness | Yusufuddin, 2008 |
| 7. | Qurs-e-Salajeet | Centaurea behen, shilajit, Accacia arabica, Calcined egg, Calcined tin,and +5 | 260 mg b.i.d. (tablet) | 15% | General weakness, spermatorrhoea, and polyurea | Yusufuddin, 2001 |
| 8. | Sharbat-e-Salajeet | Trachyspermum ammi, Amomum subulatum, Apium graveolens, Cinnamoum zeylanicum, Zingiber officianale, Cinnamomum tamala, Piper nigrum, shilajit, Sugar, and Citrus aurantifolia | Syrup 6 mL twice a day. | 4% | Spermatorrhoea | Yusufuddin, 2001 |
| 9. | Habb-e-Muqawwi Khas | Shilajit, Pistacia lentisus, Bezoar stone, Pinctada imbricata radiata, Bambusa arundinacea,and +10 | 5 g | 6% | Nervine weakness and sexual debility | Yusufuddin, 2001 |
| 10. | Habb-e-mumsik jadeed | Cannabissativa, Hyoscyamusniger, Peganum harmala, shilajit, Myristica fragrans, and +7 | 5 g | 8% | Premature ejaculation | Yusufuddin, 2001 |
| 11. | Habb-e-Nishat jadeed | Shilajit, Calcined dust of zinc, tin, led, silver, and ruby, Crocus sativus, Rosa damascena, Myristica fragrans,and +19 | 5 g | 5.5% | Sexual debility | Yusufuddin, 2001 |
| 12. | Zehbi | Melissa parviflora, Centaurea behen, Chiranthus cheiri, Salvia haematodes, shilajit, and+11 | 6 g | 8.5% | Cerebral asthenia, weakness of heart, neurasthenia, and palpitation | Yusufuddin, 2001 |
| 13. | Imsakeen | Acacia arabica, Cannabissativa, Hyoscyamusniger, Musua ferrea, shilajit, and +14 | 6 g | 4% | Premature ejaculation | Yusufuddin, 2001 |
| 14. | Majoon Nishat Angaiz | Alpinia galanga, Myristica fragrans, Tribulus terrestris, Cinnamomum cassia, shilajit,and +15 | 6 g | 1% | Sexual debility, impotency, and debility of vital organs | Yusufuddin, 2001 |
| 15. | Muqawwi mumsik | Shilajit, Cinnamoum zeylanicum, Pistacia lentisus, Myristica fragrans, Nardostachys jatamansi,and +15 | 1 g | 0.04% | Premature ejaculation and sexual debility | Yusufuddin, 2001 |
Table 8
Formulations mentioned from the Ayurvedic system of medicine.
Humic substances have the potential to be explored in all four categories, which is not a common phenomenon for all natural ingredients. Table 6 provides a list of some natural ingredients being explored in different categories. There are around 150 dietary DS-based products available globally, and research is being conducted to establish their safety profile and efficacy. Furthermore, it is also marketed as veterinary products, a unique aspect of HS, compared to other natural remedies. Its exploration as a pharmaceutical excipient is still under investigation. However, the journey so far and the way forward is not as simple as it appears. There are several reasons why HS are not explored extensively so far; this could be due to expensive raw materials (i.e., shilajit of Himalayan regions), structural complexity, and physicochemical properties (Gnananath et al., 2020). The manuscript discusses the challenges in acceptance of HS as healthcare products (prescription or dietary supplement category) generally asked by the stringent regulatory authorities worldwide. Despite successful sales as DS in the European Union (EU), United States, Commonwealth of Independent States (CIS) region, and Australia, the industry still faces tough questions during the approval (marketing authorization) process. The HS industry faces difficulties, unlike the pharmaceutical industry, because DS or NC marketing authorization in one country is difficult to be accepted as a reference for another region, although it supports the application.
2. MATERIALS AND METHODS
This study includes an extensive literature search (using different search engines, such as PubMed, Web of Science, Science Direct, Scopus, Embase, Google Scholar, and Ovid), company and regulatory agency’s websites, and personal experiences in dealing with regulatory agencies during product approval.
3. HUMIC SUBSTANCES IN INDIGENOUS MEDICINE
Traditional or folk medicine is an indispensable facet of the global healthcare system. Its practices, passed down through generations, have proven to be effective in treating a myriad of ailments (Hill, 2009). From herbal remedies to acupuncture, traditional medicine offers an alternative to conventional treatments, and it has been an integral part of healthcare for centuries (Yuan et al., 2016). Therefore, it is important to embrace and respect the value of traditional medicine in order to provide holistic healthcare to all individuals. Indian traditional and folk systems, such as Ayurveda, Unani, Siddha, acupuncture, yoga, and indigenous therapies, are highly effective (Rizvi et al., 2022). According to the World Health Organization (WHO, 2023), an overwhelming number of countries, or 88% of the 170 member states, use traditional medicine.
Shilajit is used in the Indian subcontinent’s traditional medicine as a rejuvenator and an adaptogen (Andrade et al., 2023). It is also known as mumie, mumijo, asphaltum, punjabinum, arakul dshibal, and Hajar-ul-musa. In traditional medicine of Tibet, it is called zhaxun, meaning the “Juice of rock” or “the essence of rock” (Frolova & Kiseleva, 1996). The word “shilajit” is derived from two Sanskrit words: shila, meaning rock, and jeet, meaning win, which collectively means “conqueror of mountains” and “destroyer of weakness.” According to old traditional healers, there is hardly any curable disease that cannot be controlled or cured with shilajit (Agarwal et al., 2007). Shilajit is predominantly composed of paleohumus (80–85%), defined as “fully decomposed organic matter,” mainly derived from fossils that have been present inside the layers of rocks for many years, subjected to high temperature and pressure, ultimately metamorphosing into this material (Ghosal et al., 1997). It loses its viscosity in the summer season and oozes out from the rocks.
Chemically, shilajit contains gums, albuminoids, traces of resin and fatty acid, and a large quantity of benzoic and hippuric acids and their salts. The main active substances among all the medicinal compounds present in it are benzoic acid and benzoates (Kamgar et al., 2023), including bioactive oxygenated dibenzo-α-pyrones, tirucallane triterpenes, phenolic lipids, and small amount of tannoids. Shilajit, obtained from different sources, is a complex heterogeneous mixture, rather than a well-defined natural product and has varying amounts of both FA and HA as well as other compounds; although the overall structures of these compounds are poorly defined. However, now it has been standardized on the basis of its major organic constituents that shilajit mainly includes organic matter, HA, FA, and volatile and fat soluble components (Ghosal et al., 1995).
In the given manuscript, two major Indian systems of medicine (Ayurveda and Unani) are included. Shilajit, a major raw material of the Indian subcontinent, mainly contains HS. Globally, FA is often extracted from other deposits, such as peat lignite, leonardite, and coal. On the other hand, FA is also found in natural water sources, such as rivers, lakes, and streams.
As a prescription product, also known as a prescription medication or drug, shilajit is legally available only with a valid prescription from a licensed healthcare professional. Such medications are typically regulated by health authorities to ensure their safe and appropriate usage. Shilajit is the only humic substance used in different products of Indian system of medicine. The products are included in the formulary and pharmacopoeia of indigenous medicines (see Tables 7 and 8).
4. HUMIC SUBSTANCES AS DIETARY SUPPLEMENTS IN GLOBAL MARKET
DS or NC are included in the category of non-prescription products. They have no label claims for any therapeutic activity and are sold without prescription (a type of over-the-counter [OTC] products). This is the most common category of HS-based products and are available globally and even in some of the most stringent regulatory framework countries. Companies in this category typically operate on a small scale. The products are available in the form of oral solids (tablets, capsules, caplets, bars, and powder), oral liquids (solution and suspension), and topical products. Table 3 and Table 4 present some surprising facts about the available products, such as the following:
There is a significant variation in the dose of HS up to 4.8-g FA per day for adults to 300 mg for children.
There is a substantial variation in the regimen of consumption. The recommended regimen is up to 6 months in some orally consumed products, while in some labels, the recommended duration hasn’t been mentioned, allowing usage for unlimited period.
There is no quantification of HS in some of the products, and the daily intake of HS is not quantified for both adult and children.
5. ROLE OF HUMIC SUBSTANCES AS COSMECEUTICALS
Cosmeceuticals are the cosmetics exhibiting medicinal properties because of the existence of bioactive components corresponding to medicinal benefits (Choi & Berson, 2006). The distinct comparison between an inert cosmetic and a cosmeceutical can be relatively befitting, as an inert cosmetic is meant for aesthetic augmentation and quality enhancement whereas cosmeceutical is perceived for pharmacological healing (Kligman, 2000).
Humic substances have immense therapeutic potential, and hence cosmetics that include HS fabricate a significant proportion of cosmeceuticals. Globally, products constituting a specified combination of salicylic acid and HA in face cleansing and peeling creams serve manifold purposes, with skin enhancement being main function. The role of HS as bio-fertilizers and shoot growth-promoting agents is also worth acknowledging. HS are also often combined with herbal phytoconstituents, such as aloe, apple cider, and epigallocatechin-3-gallate (EGCG), to constitute veterinary and infant cosmeceuticals that have wide applications across the globe. Advantages of HS in skin care are as follows:
Promoting cellular uptake of nutrients.
Having established detoxification properties.
Being of natural origin with minimum involvement of chemicals during extraction.
Claiming properties, such as healing, antioxidant, anti-aging, and astringent.
Today, mud, mineral and peat baths, as well as various natural packs and face masks, are offered in spas and wellness centers worldwide. Balneotherapy (a traditional bathing therapy) involves immersing a subject in natural mineral water or mineral-laden mud. The active substances in these mud baths are HS. For this reason, interest has increased in the use of HA and FA in dermatology and cosmetics.
6. HUMIC SUBSTANCES IN MODERN MEDICINE
Carbohydrate-derived FA (CHD-FA) is one of the products of this category that has undergone clinical trials to establish safety and efficacy for the topical treatment of eczema/dermetitis. Preclinical safety and efficacy data are available for this product with different indications. Manufacturers claim it to be a better FA than the naturally obtained HS. However, researchers working on HS find it debatable to be called CHD-FA, as the process of obtaining it does not adhere to widely accepted definitions.
Several clinical trials have been conducted and are ongoing to prove the therapeutic efficacy and potential of HA and FA (Table 9). An investigational randomized, double-blind clinical trial, comprising 15 males, was conducted to evaluate comparable efficiency with a placebo as a control measure for the treatment of erectile dysfunction and to enhance sexual libido. Results of the study showed an improvement in the sexual health scores as well as augmentation in sexual quality score (NCT02794454) (Sandip & Shirish, 2016).
Table 9
Clinical trial data was obtained from different clinical trial portals.
| S. No. | Clinical trial ID | Title | Country | Phase | Recruitment status | Sample size | Sponsor | References |
|---|---|---|---|---|---|---|---|---|
| 1. | IRCT20191216045758N1 | Evaluation of the effect of the drug “Mumiaee” on the union period of distal radius fractures: a randomized clinical trial | Iran | 2–3 | Recruiting | 44 | Mashhad University of Medical Sciences, Iran | Ali, 2020 |
| 2. | IRCT20200617047808N1 | Effects of oral shilajit tablet on sexual function and sexual quality of life among married women of reproductive age: a triple blind; randomized; controlled; clinical trial study | Iran | 1 | Recruitment complete | 100 | Shahid Beheshti University of Medical Sciences, Iran | Mosavi et al., 2023 |
| 3. | UMIN000041443 | Exploratory test on the influence of continuous intake of FA on the body | Japan | - | Complete | 36 | Akasaka Family Clinic, Japan | Mitsuko, 2020 |
| 4. | UMIN000023456 | Randomized control study for the effect of dentifrice, including mastic and FA on periodontal symptoms | Japan | - | Pending | 30 | The Ministry of Welfare and Labor, Japan | Hisashi, 2016 |
| 5. | DRKS00005183 | Irritable bowel syndrome—improvement to the clinical situation by the use of HA | Germany and Austria | - | Recruiting stopped | 78 | WH Pharmawerk Weinböhla GmbH, Germany | Svent, 2019 |
| 6. | CTRI/2012/01/002324 | The investigation of FH0210 (carbohydrate-derived FA [CHD-FA] in a formulated wellness drink) for self-medication to prolong pre-art phase in HIV positive subjects | India | 3 | Recruiting stopped | 260 | Vihoton Pty Ltd., South Africa | Botes et al., 2018 |
| 7. | CTRI/2012/06/002712 | Randomized placebo controlled double blind clinical study of shilajit rasayana compound in the management of diabetic polyneuropathy | India | 2 | Recruiting stopped | 60 | Institute for Post Graduate Teaching Research in Ayurveda, Jamnagar, India | Baghel, 2012 |
| 8. | CTRI/2018/04/013529 | Evaluation of the effect of aqueous extract of shilajit on bone health and its biomarkers in post-menopausal women | India | - | Complete | 60 | Nizams Institute of Medical Sciences, India | Pingali & Nutalapati, 2022 |
| 9. | NCT03372109 | Evaluate the effects of repeated periods of modified fasting to support healthy natural weight management and prevention of weight gain | USA | - | Complete | 23 | Supplement Formulators, Inc., USA | Joyal, 2017 |
| 10. | NCT02026414 | Effects of PrimaVie and exercise training on human skeletal muscle (PrimaVie) | USA | - | Not yet recruiting | 40 | SanniRaju, Ohio State University, USA | Raju, 2014 |
| 11. | NCT00381849 | Use of an herbal preparation to prevent and dissolve kidney stones | USA | 1–2 | Complete | 20 | Mayo Clinic, USA | Erickson, 2006 |
| 12. | NCT02794454 | A Study to evaluate efficacy of investigational product HeezOn Ultra-1/HeezOn Ultra-2 on male sexual health | USA | - | Complete | 15 | Vedic Lifesciences Pvt. Ltd., India | Sandip & Shirish, 2016 |
An interventional study intended to assess the effectiveness of shilajit pill in sexually active married women was conducted in Tarbiat Modares School of Medical Sciences, Nasr, Tehran. Its results were based an the analysis of a list of questionnaires (IRCT20200617047808N1) (Mosavi et al., 2023). This study concluded that Shilajit pills significantly improved sexual function in sexually active married women but did not have a significant effect on their overall sexual quality of life. In Japan, a novel exploratory clinical trial focused on identifying the effects on sustained intake of FA (UMIN000041443) (Mitsuko, 2020). Germany and Austria collaboratively conducted a randomized clinical trial on irritable bowel syndrome and the consequence of utilizing HA as a clinical intervention (DRKS00005183) (Haufe et al., 2021). Both these trials focused on the health effects of sustained intake or intervention with specific agents (FA and HA). The Japanese trial was exploratory and targeted general health parameters, while the German-Austrian trial was randomized and focused specifically on IBS. Both these trials showed positive, statistically significant outcomes for their respective interventions.
In India, a clinical investigation was carried out on HIV+ patients to study the effects of extended self-administration of CHD-FA wellness drink on HIV (CTRI/2012/01/002324) (Botes et al., 2018) study revealed that HIV+ patients safely and tolerably self-administered the CHD-FA wellness drink over an extended period. A randomized placebo-controlled, double-blind clinical study was conducted in India on shilajit for the management of diabetic polyneuropathy to substantiate the hypoglycemic potential of HS (CTRI/2012/06/002712) (Baghel, 2012) and showed that shilajit can be utilized for diabetic polyneuropathy and found it significantly improved blood glucose control and neuropathic symptoms when compared to a placebo.
A study in India was jointly conducted by Sawai Man Singh Medical College, Jaipur, and the National Institute of Ayurveda, Jaipur, to determine the impact of shilajit on the blood chemistry of healthy human volunteers. The results showed that administering 2 g of shilajit for 45 days did not significantly affect physical parameters, such as blood pressure, pulse rate, and body weight, nor did it caused any changes in hematological parameters. There was a significant reduction in serum triglycerides and serum cholesterol, accompanied by an improvement in high-density lipoprotein (HDL) cholesterol levels. Additionally, shilajit enhanced the antioxidant status in all participants. The study’s results suggested that shilajit has hypolipidemic and potent antioxidant properties (Sharma et al., 2003).
Multiple clinical trials have been conducted to validate and evaluate the effects of FA on male sexual health, and the ability of such compounds to treat male infertility and associated conditions, such as oligozoospermia and erectile dysfunction. Traditionally, it has been witnessed that HS, mainly FA, are employed in the treatment of arthritis and other bone ailments. Clinical trials were conducted to authenticate the osteo-strengthening therapeutic potential of HS compounds. Intricate elucidation of these clinical trials are provided in Table 9.
FA possesses different properties that can be exploited as excipients in conventional and novel drug delivery systems (such as 505(b)(2) application to the US Food and Drug Administration [US FDA] and hybrid application to the European Medicines Agency [EMA]). There are some literature reports of FA exploration in drug delivery (Mirza et al., 2011c, 2016). The impediments encountered and the solutions are discussed in Mirza (2018).
7. GLOBAL PATENT STATUS OF HUMIC SUBSTANCES
The therapeutic potential of HS is widely directed globally. HS are efficiently used as skin cleansing and acne-preventive agent, patented (CN/1219507/C) by Xuesong et al., 2005. it is also used in hypoglycaemic preparations (e.g., sodium fulvate) and oral hygiene products (e.g., FA), patented (CN/101744844/B) by Baocai et al., 2012. The most crucial implication of HS in the current scenario is their inclusion as active NC formulations, either singly or in combination with other immunity-enhancing food supplements with disease preventive actions, as observed in ashwagandha- and shilajit-patented formulations. FA itself is elucidating the complications of systematized drug delivery. Purified FA is used as a carrier in the formation of a patented water-soluble drug delivery system (shilajit formulation; EP/3672578/A1) (Roger et al., 2020). Along with the amalgamation of Chinese medicine, edible-grade FA and excipients, a patented FA beverage is developed with multifactorial advantages, such as enhancing immune response and heart health, lowering of blood glucose levels, etc. (CN/103564587/B) (Min and Huifen, 2015). This patented adaptogenic herbal chewing gum comprises shilajit, ashwgandha, citrulline malate, Siberian ginseng, L-tyrosine, Ganoderma lucidum (Reishi), Hericium erinaceus (Lion’s mane), coconut oil, and resveratrol, and has multiple health benefits (Roger et al., 2020). The epidemiological cluster accessing such products can be authenticated by intricately introspecting patent information as well as marketing data. An elaborate description of several such essential patents is given in Table 10.
Table 10
Patents related to healthcare based on humic substances in various regions have been retrieved using different search portals.
| S. No. | Title of patent | Application number/grant number | Details | References |
|---|---|---|---|---|
| 1. | Combination containing FA for cleaning skin | CN/1219507/C | The effective amount of FA accounts for 0.5–5% of the total weight of the composition. The end product, liposomes, is obtained by incorporating phosphatidyl choline as a wall material to embed FA. It can be further developed into various personal hygienic products, such as bath lotion, handwashing solution, etc. | Xuesong et al., 2005 |
| 2. | Anti-acne mask containing FA and its preparation method | CN/103520072/A | The anti-acne mask was formulated using FA, pearl powder, motherwort, lavender, skin moisturizing oil, a thickening agent, and a surfactant as raw materials. The addition of natural FA, known for its anti-inflammatory, analgesic, and endocrine-regulating properties, enhances the mask’s effectiveness against acne. This mask is mild, causing no skin irritation or adverse effects. It effectively eliminates acne marks, aids in skin repair, is easy to apply, and ensures controllable quality. | Baocai et al., 2014 |
| 3. | FA or sodium fulvate substance with hypoglycemic function | CN/101744844/B | The oral solution’s composition, expressed as a percentage by weight, includes 1–10% of FA or sodium fulvate, 0.5–10% of polysaccharide sulfate, and the remaining content as water. The healthcare product containing FA or sodium fulvate demonstrates a significant reduction in blood sugar, total cholesterol, and triglyceride levels in individuals with diabetes. Additionally, it enhances oral carbohydrate tolerance and regulates blood sugar levels. | Baocai et al., 2012 |
| 4. | A kind of nano-scale FA peat facial mask and its preparation method thereof | CN/106726655/A | The composition consists of the following proportions by weight: FA 15.5–16.2%, peat 55.8–62.5%, starch 9.0–10.8%, deionized water 8–10%, glycerine 1.5–3.5%, and sodium benzoate 0.01–0.035%. The process is characterized by its simplicity, low cost, and straightforward process control. | Pengyuan et al., 2017 |
| 5. | FA toothpaste and its preparation method thereof | CN/105686979/B | The toothpaste includes 1.0–20.0 parts of FA, and 0.03–3 parts of bacteriostatic agent in mass fraction. The use of this toothpaste inhibits the intraoral acid production of Lactobacillus bulgaricus, Streptococcus thermophilus, and Lactobacillus acidophilusprotecting teeth from acid erosion and enamel damage. | Guanjun et al., 2019 |
| 6. | Natural FA compound healthcare beverage and its preparation method | CN/103564587/B | The beverage comprises the following components by weight percentage: 0.1–0.6% of edible-grade natural FA, 0.05–0.2% of extracts from traditional Chinese medicine, 2–6% of sweetening agent, 0.01–0.5% of acidulant, 0.01–0.15% of stabilizer, with the balance being water.The healthcare beverage offers functions, such as preventing and treating gastrointestinal diseases, preventing tumors, improving the human body immunity, reducing blood glucose and blood fat, enhancing appetite, and promote better sleep. Notably, it is free from adverse effects when consumed over an extended period, making it an ideal healthcare beverage. | Min & Huifen, 2015 |
| 7. | Preparation method of FA nano-silver gel | CN/107398562/B | The present invention uses a reducing agent and stabilizer derived from the natural FA extraction solution to synthesize nano-silver grains using a microwave-assisted synthetic method. This process is simple and rapid, with the notable advantage of not requiring the addition of any chemical reagents throughout the preparation process. It stands out for its environment-protective, convenient, and efficiency. | Ning & Weihong, 2019 |
| 8. | Delivery system for pharmaceutical, nutritional, and cosmetic ingredients | EP/1435982/A4 | A stable, water-soluble delivery system is presented, featuring a purified shilajit composition with a preference for at least 40% by weight of a carrier, which is purified FA. This carrier is characterized by a sponge-like structure punctured by voids of about 200–1000 Å in diameter, with a Mn molecular weight of about 700–2500. The delivery system also includes an effective amount of an active pharmaceutical, nutritional, or cosmetic ingredient added to the carrier, filling the voids within. | Shibnath, 2010 |
| 9. | Promoting muscle building and repair and treating disorders related to collagen and pertinent proteins by using shilajit | JP/2020073552/B1 | The method comprises administering a therapeutically effective amount of shilajit or its individual components, or a combination of two or more of these components. Through this administration, the mammal’s body synthesizes new collagen and/or one or more proteins selected from the group consisting of tenascin, decorin, elastin, myoferlin, fibrillin, and fibronectin. | Sen, 2017 |
Overall, the reviewed trials highlight a variety of potential applications of HS, particularly shilajit and FA. Most studies report positive outcomes in the areas such as bone health, sexual functioning, digestive health, and muscle performance. The future clinical trials should focus on larger sample sizes, diverse populations, and longer follow-up periods to confirm the efficacy and safety of these substances, ensuring their reliable integration into therapeutic practices.
8. COSMETIC AND PHARMACEUTICAL APPLICATIONS OF HUMIC SUBSTANCES
The most widely used category of HS is DS or NC. In countries where HS are neither indigenous nor part of the traditional system of medicines, HS products in the market face some common regulatory queries as mentioned in Section 10. The exporters to these countries also encounter queries on similar lines. The earlier studies (Jain et al., 2020; Khan et al., 2022; Mirza et al., 2011a; Mirza et al., 2011b) have demonstrated that both FA and HA function as solubilizing agents by condensing pharmaceutical and cosmetic-active ingredients within their micelle-like structures. These structures, with their core voids, enhance water solubility through the formation of inclusion complexes. Additionally, the phenolic groups present in HS act as electron donors, neutralizing free radicals and preventing chain reactions (de Melo et al., 2016). These antioxidant and solubilizing properties of HS make them valuable constituents of both cosmetic and pharmaceutical industries. Similar to β-cyclodextrin, HS also contain void spaces and can form inclusion complexes. As a result, our research team also developed a ternary complex involving hydrolyzed Bacopa monnieri, FA, and hydrogenated soy lecithin to improve the entrapment efficiency of phospholipid complexes, akin to the drug-in-cyclodextrin-in-liposome system (Gnananath et al., 2022).
9. EXPLORING THE IMPACT AND MECHANISM OF HUMIC SUBSTANCES ON HUMAN HEALTH AND AGRICULTURE
Humic substances are vital organic compounds found in both terrestrial and aquatic ecosystems, playing a crucial role in environmental sustainability and contributing to animal and human well-being. Their complex structures and formation processes influence various functions, including nutrient cycling, metal ion mobilization, soil fertility, and pollution management. In addition to their environmental significance, HS have shown potential health benefits, such as enhancing gut health and supporting detoxification processes. As mentioned previously, understanding these substances requires a cross-disciplinary approach, involving expertise in humic chemistry, regulatory affairs, and product development to effectively apply them as excipients or NCs (Gnananath et al., 2020). Ongoing research in biochemistry, molecular biology, and environmental science would undoubtedly enhance our comprehension of HS, highlighting their essential role in balancing of the ecosystem and in promoting both animal and human health.
10. GENERAL REGULATORY QUERIES ASSOCIATED WITH HUMIC SUBSTANCES
Some common regulatory queries are mentioned below.
Safety concerns on the recommended doses.
Complete list of ingredients/elements present in the preparation.
Safety concerns about the quantity of natural trace elements present.
Submission of safety and efficacy reports of the humic materials to regulatory agencies is often complicated by different in the origin, location, or manufacturering processes of the materials. Also, most of these existing studies are not designed as per regulatory guidelines.
Improper quality-related data.
Shelf life of the finished product.
Some queries are region-specific, such as local ecological policy on bottle and packaging recycling, and the enivronmental impact of land usage related to material extraction, etc.
Some of the above-mentioned queries are easily addressed (in a cost-effective manner) by generating quality-related data (i.e., in vitro data); however, safety and efficacy establishments require a full battery of studies on animals (in some cases humans too), which is undoubtedly a major investment. DS- or NC-based companies have their own apprehension about investment, primarily considering the size of the business involved. One major concern is the lack of quality standards, specifications, or monographs of HS for human-grade products. Their absence hinders the growth of natural HS in the realm of human-grade products. Some regulatory agencies also request a complete list of ingredients present. While intentionally added ingredients are generally fewer, the associated complexes/minerals/elements are numerous. Though the complete list of analysis can be given to the agency as a one-time report, companies refrain from including it in batch release specifications or certificates of analysis because it may increase production cost, ultimately affecting the retail price of final product. The analysis and quantification of HS is itself a challenging task. The Lamar method is a well-accepted method (Lamar et al., 2014) for quantification, but it is time-consuming and the availability of DAX-8 resin is not so easy to come by. There are technical challenges in developing chromatographic or spectroscopic quantification methods for HS.
The good part is that HS-based research is in progress globally. A well-coordinated effort may address the issues more efficiently. If a sound regulatory strategy is drawn (such as 505(b)(2) application of the US FDA), the bridging data can be generated between different datasets available in public domain, and strong safety and efficacy claims can be established, which is definitely a multidisciplinary assignment. Global organizations, such as International Humic Substances Society and Humic Products Trade Association, can take a lead in channelizing global efforts. Considering the inherent variability observed in materials, specifications can be categorized into different grades and sub-grades.
11. CONCLUSION AND EXPERT PERSPECTIVES ON HUMIC SUBSTANCES
Undoubtedly, although HS have achieved great success in irrigation as fertilizers and for soil health, and their various pharmacological or therapeutic potentials for human health are to be explored. This review synthesized the current state of research on HS, providing insights into their applications across multiple industries. However, so far, the success is not up to expectations. The reason definitely lies in the complexity of the moiety and lack of well-coordinated efforts. In general, the commercial players involved are small business groups, and funding for its research is also an impediment. There are examples where a substance can be successfully explored if experts from regulatory, analytical, pharmacology, and chemical science are brought together. In case of lack of funds, a long-term strategy can be chalked out, and academia and non-profit organizations can be brought on board. However, the responsibility to execute the strategy should lie with a particular group or organization.
Humic acid and FA have emerged as a prominent areas of research because of their diverse applications in health, NCs, pharmaceuticals, and cosmetics. Beyond their agricultural benefits, HS are increasingly recognized for their bioactive properties that make them suitable for various therapeutic and cosmetic applications.
The bioactivity of HS stems from their complex molecular composition, which varies depending on their natural source. These molecules contain an array of functional groups that interact with cellular components, enabling applications in drug delivery and skincare, where they act as natural antioxidants, antimicrobial agents, and enhancers of nutrient bioavailability.
Despite the potential health benefits of HS, there remain regulatory and scientific challenges to their widespread adoption, especially in medicinal and food applications. Many studies confirmed their efficacy as functional excipients that improve the solubility and stability of biopharmaceutics classification system (BCS) class ıı and ıv drugs.
Their complex and variable composition, influenced by source and extraction methods, complicates regulatory approval and standardized application to therapeutics.
Humic substances have not yet achieved the “generally recognized as safe” status. Toxicological studies have been conducted to assess the safety profile of HS, yet the lack of uniformity in HS samples—arising from differences in molecular size, functional groups, and elemental composition—necessitates further research to establish consistent standards.
A multidisciplinary approach combining humic chemistry, toxicology, pharmacology, and regulatory science is essential to streamline the incorporation of HS in health products while ensuring their safety and efficacy.
This review compiled the most significant findings from clinical studies and highlighted key patents, illustrating innovative applications that showcase HS as potential bioactive agents. By addressing both therapeutic and commercial potential of HS, this review aspired to foster cross-disciplinary collaboration among scientists, healthcare professionals, and industry stakeholders. The consolidated information presented here underlines the need for continued research to unlock the full potential of HS, paving the way for new, safe, and effective products in the health and wellness sectors.
AUTHOR CONTRIBUTIONS
Mohd. Aamir Mirza: research concept and design, data analysis and interpretation, and writing of the article; Rajat Goel and Somaan Lareb: collection and/or assembly of data; Uzair Ali: writing of the article; Aslam Siddiqui: data analysis and interpretation; Zeenat Iqbal: critical revision of the article; Kattamanchi Gnananath: data analysis and interpretation, and critical revision of the article; Mohammed Aslam: writing of the article; Noor Alam: data analysis and interpretation; and Vinay Bharadwaj Tatipamula: writing and critical revision of the article. The final manuscript was verified and approved by all the authors.