1. INTRODUCTION
The common butterbur (Petasites hybridus L.) is a medicinal plant belonging to the Asteraceae family, primarily native to Europe, Southeast Asia, and certain regions of North Africa (Aydın et al., 2013). I It has been traditionally used to treat a wide range of medical conditions, including migraine, hypertension, bronchial asthma, and allergic rhinitis (Blosa et al., 2021; Lipton et al., 2004; Sun-Edelstein and Mauskop, 2009; Thomet and Simon, 2002).
The active constituents of butterbur are sesquiterpene compounds, namely petasins, which are predominantly found in the roots. Studies have shown that the anti-inflammatory and antiallergic activities of petasins are based on the inhibition of leukotriene and histamine activity (Blosa et al., 2021; Schapowal, 2005; Thomet et al., 2002; Thomet and Simon, 2002). Although Petasites hybridus extracts selectively inhibited COX-2 and prostaglandin expression in microglial cells, this effect was not directly correlated with petasin content (Fiebich et al., 2005). Petasins have also been shown to inhibit calcium channels, making them effective in the treatment of hypertension and migraine (Guo et al., 2020; Sheykhzade et al., 2008; Wang et al., 2010). Furthermore, studies have demonstrated that petasins possess anti-tumor activities (Guo et al., 2020), and that butterbur root extract exhibits anti-cancer effects on breast cancer cell line—while sparing non-cancerous cells—primarily through the induction of apoptosis, necrosis, and oxidative stress (Apostolova et al., 2023). A recent review discussed the pharmacology, safety, and clinical efficacy of butterbur in the prevention of migraine attacks (Borlak et al., 2022).
Since a dietary supplement containing common butterbur root extract may be beneficial for all the conditions mentioned above, developing a standardized and stable finished product of butterbur extract is essential to ensure therapeutic efficacy (Kim et al., 2019). Therefore, this study aimed to demonstrate the development process of softgel capsules containing butterbur root extract, including the formulation and manufacturing scheme to produce a stable, effective, and aesthetically appealing softgel dosage form. The study also focused on evaluating the stability of the finished product—softgel capsules containing butterbur—and establishing an analytical assay method for measuring petasins within these capsules. A high-performance liquid chromatography (HPLC) assay method was selected to analyze petasins, the key reference compounds in the softgel capsules, with stability testing performed under both accelerated and real-time conditions.
2. METHODS
2.1. Purple butterbur root extract
Purple butterbur root extract was purchased from RIA International, New Jersey, USA. According to the supplier, the roots were harvested in Germany during late summer to early autumn, when petasin levels were at their peak, then dried at temperatures below 40°C and ground to a 40–60 mesh powder. Extraction was carried out using 80% ethanol at a plant-to-solvent ratio of 1:10 (w/v), employing ultrasonic-assisted extraction at 45°C for 30–60 min, followed by reflux extraction at 65°C for 2–3 h. The extract was subsequently filtered through a 0.45 µm filter and concentrated under vacuum at 40–50°C using a rotary evaporator. The resulting extract underwent charcoal filtration to remove pyrrolizidine alkaloids. After this treatment, the extract was converted into powder form using spray drying at temperatures below 50°C. The butterbur extract was then standardized to contain 15% total petasins through fractionation with ethyl acetate and water, followed by quantification of petasins (Table 1).
Table 1
Specifications and testing methods for the butterbur root extract.
2.2. Formulation, preparation of softgel capsules, and manufacturing process
The manufacturing and quality control processes of the butterbur softgel capsules are outlined in Figure 1 and Table 1. Briefly, the shell material is prepared using gelatin, glycerin, purified water, titanium dioxide, FD&C Red No. 40, and FD&C Yellow No. 6. The mixture is heated and stirred until fully dissolved, forming the gelatin solution (Figure 1). Purple butterbur root extract (50 mg) is combined with soybean oil, beeswax yellow, and lecithin to create a uniform fill mixture (Figure 1). The fill material and the gelatin solution are then combined and stirred thoroughly to ensure even distribution of the extract and excipients. Next, the gelatin solution is fed into a rotary die encapsulation machine to form the softgel shells, while the fill material is simultaneously encapsulated within the gelatin shells. The filled softgel capsules are sealed and cut to the desired size and shape. Quality control steps are conducted on the gelatin mix, during mixing, and after softgel encapsulation using specified criteria and test methods, as outlined in Table 2.
Figure 1
. Manufacturing process of butterbur softgel capsules. Quality control (QC) steps were performed as outlined in Table 1.
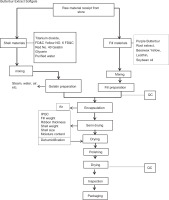
Table 2
Quality control parameters for butterbur softgel capsules.
2.3. Drying and conditioning
The softgel capsules are dried under controlled temperature and humidity conditions to achieve the desired moisture content and hardness. After drying, the capsules are conditioned to ensure uniformity and stability (Figure 1, Table 2).
2.4. Identification of Purple Butterbur Root Extract
The purple butterbur root extract in the finished product was identified from the content of no less than 10 capsules using thin layer chromatography (TLC) with a 0.50-mm layer of chromatographic silica gel mixture. Following extraction by heating under reflux for 20 minutes with about 40 ml of light petroleum on a water bath, the filtrate was concentrated to approximately 1 ml. A volume of 20 µL of both the standard and sample solutions was applied to the TLC plate, with chloroform used as the mobile phase. The plates were sprayed with a mixture of 0.5 ml anisaldehyde and 10 ml glacial acetic acid, then heated at 120°C for 7–10 minutes. The plates were examined under UV light at 230 nm. The sesquiterpenes petasin and isopetasin were detected in the Rf range of 0.4–0.45 as green-blue fluorescent zones.
2.5. Capsule Weight and fill content weight
Twenty softgel capsules were randomly selected and weighed, and the average weight was recorded. Weight variation was calculated according to USP-NF <2091> Weight Variation of Dietary Supplements. The average fill content was measured by weighing 10 intact capsules selected at random; each capsule was then opened, and its contents were completely recovered using a solvent. The empty shells were kept at room temperature for 30 minutes to allow the solvent to evaporate, after which each shell was weighed. The average shell weight was calculated, and the average fill weight content was determined by subtracting the average shell weight from the average capsule weight.
2.6. Rupture time of capsules
The rupture time of softgel capsules was determined according to USP-NF <2040> Disintegration of Dietary Supplements. Briefly, each capsule was placed in a dissolution vessel containing 500 ml of water, and the dissolution apparatus was run for 15 minutes. Rupture time was recorded within this 15-minute period.
2.8. Analytical method of petasins
2.8.1. Analysis
Dry and chopped rhizomes of P. hybridus (Freising, Germany) or samples from the softgel capsules were collected and processed as follows. Extraction was performed using a stoichiometric plant-to-solvent ratio of 1:10 (w/v). The material was steeped in ethanol at 38°C for 1 hour, and the resulting extract was filtered through filter paper and a 0.2 µm RC membrane filter at room temperature. The filtrate exhibited a yellowish color with a characteristic butterbur scent.
Chromatographic fractionation of the filtrate was carried out using an HPLC-UV system (Merck-Hitachi HPLC system, Tokyo, Japan) equipped with an auto-sampler, binary pump L-7100, and Diode-Array Detector L-7450, controlled by LaChrom D-7000 HPLC-System-Manager. Separation was performed on an Agilent Zorbax Eclipse XDB-C18 reversed-phase column (100 mm × 3.0 mm i.d., 3.5 µm particle size) using elution solvents of methanol–ammonium acetate (10 mM aq.) in a 90:10 (v/v) ratio and ammonium acetate (10 mM aq.)–methanol in a 90:10 (v/v) ratio at pH 7.4 as the organic and aqueous phases, respectively, at a flow rate of 0.4 mL/min. Chromatograms were recorded at a wavelength of 230 nm. Fractions were collected using a Pharmacia LKB Frac-100 auto-fraction sampler (Uppsala, Sweden) at 1-minute intervals. From each fraction, a 15 µL volume was injected and repeated four times to increase the total amount of collected drug constituents. Fractions corresponding to a chromatographic mobile phase volume of 1.6 mL (i.e., 4 × 0.4 mL) were evaporated at room temperature under continuous nitrogen flow. The mobile phase consisted of methanol–ammonium acetate (10 mM aq.) in a 90:10 (v/v) ratio and ammonium acetate (10 mM aq.)–methanol in a 90:10 (v/v) ratio at pH 7.4 as the organic and aqueous phases, respectively. The standard solution was prepared from pure petasin (98.0%) purchased from TLC Pharmaceutical Standards.
The percentage of the labeled amount of petasin in the capsule was calculated using the following equation:
where
rU peak area of petasins from the Sample solution
rS peak area of petasins RS from the Standard solution
CS concentration of petasins RS in the Standard solution (mg/mL)
CU nominal concentration of petasins in the Sample solution (mg/mL)
The acceptance criterium was not less than (NLT) 15.0% of petasin of the labeled amount of purple butterbur root extract.
2.8.2. Validation
Accuracy was determined using five different levels of the working concentration, with two replicates for each concentration level. The calculated petasin content was compared to the percent recovery for each preparation against the standard solution. The percent recovery at each level was required to be between 90% and 110%.
Precision was determined by repeatability (intraday) precision and intermediate (interday) precision. Repeatability was evaluated by assaying six determinations at 100% of the working concentration, using samples from the same batch of the finished product, and calculating the relative standard deviation (RSD) for the six results. Intermediate precision was assessed by comparing the results of repeated assays performed on different days. The % RSD should not exceed 2%.
Linearity was determined using five different solutions covering a concentration range of 80% to 120%. Each solution was measured individually, and the results were plotted against the actual concentrations. The method should exhibit linearity within the desired range, with a correlation coefficient (R) of 0.99 or higher.
2.9. Stability
Stability studies on three lots of the finished product (11908S) were conducted over a 24-month period under real-time conditions of 30ºC ± 2 and 65% ± 5% RH, and over a 6-month period under accelerated conditions of 40ºC ± 2 and 75% ± 5% RH. The stability assessments included average fill weight, weight variation, rupture time, assay of petasins, and microbial testing.
3. RESULTS AND DISCUSSION
3.1. Chemical composition of the finished product softgel capsules
Each softgel capsule contains 50 mg of purple butterbur root extract, lecithin (20 mg), soybean oil (185 mg), and beeswax yellow (10 mg) as inactive ingredients (Table 3). The shell ingredients consist of gelatin, glycerin, titanium dioxide, coloring agents, and purified water.
Table 3
Description and composition of each softgel capsule.
The composition of each capsule was designed to ensure that the butterbur extract is emulsified in an oil vehicle and that the product remains stable for a long period of time (Table 3) (Szuhaj, 2003). Lecithin is known to reduce viscosity and prevent adhesion of sticky products, whereas titanium dioxide in the shell protects the capsule ingredients from light-induced degradation and heat (Blundell et al., 2022; Szuhaj, 2003). Since sesquiterpenes are the active ingredients in butterbur extracts and have poor solubility (Disch et al., 2018), the gelatin shell content with lecithin and a lipophilic vehicle were used as a drug-dosage form to increase the bioavailability of sesquiterpenes, ultimately resulting in high dosage accuracy and uniformity (Damian et al., 2021). Furthermore, adding glycerin as a plasticizer forms stable thermoreversible gel networks and lowers moisture resistance, especially in oil-based fill formulations (Naharros-Molinero et al., 2023).
3.2. Petasin Assay validation
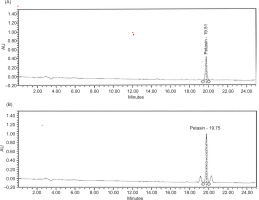
An HPLC method was developed and validated according to the definitions of ICH guideline Q2 (R2) (ICH, 2005). The petasin chromatogram extracted from butterbur was equivalent to the petasin standard solution with regard to the retention time of 19.75 min (Figure 2A and B).
Figure 2
. Chromatogram of petasin in the standard solution (A) and extracted from butterbur (B), both showing a retention times of 19.75 min.
The accuracy of the analytical method for petasin extracted from the capsules was validated using five different working concentrations ranging from 6 to 9 mg/mL, corresponding to 80% to 120% of the target concentration of 7.5 mg/mL. The results showed percentage recovery values between 101.3% and 105.15% (Table 4). Precision was validated by two different analysts on two separate days, each performing six runs per day. The intraday and interday %RSD values ranged from 0.29 to 0.31 and 0.39 to 0.79, respectively (Table 5). Linearity, based on measurements of five petasin-containing solutions, yielded a correlation coefficient (R2) of 0.9994 (Figure 3). These results were all within acceptable limits.
Figure 3
. Correlation between the measured and added petasin extracted from the softgel capsules. The relationship linear, with a correlation coefficient (R2) > 0.99 and a regression equation of y = 1.164x + 0.842.
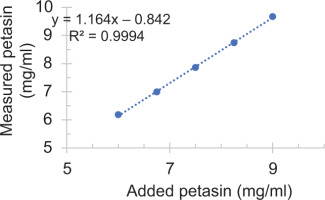
Table 4
Percentage recovery of petasins extracted from the softgel capsules.
Table 5
Precision analysis of petasin extracted from the softgel capsules.
3.3. Realtime and accelerated stability of the softgel capsules
Stability studies for three lots of the finished product (11908S) were initiated under real-time conditions of 30ºC ± 2 and 65% ± 5% relative humidity, and under accelerated conditions of 40ºC ± 2 and 75% ± 5% relative humidity (ICH, 2003). The stability data demonstrated that the softgel capsules (11908S) remained within specifications throughout the study under both conditions (Tables 6 and 7). For instance, the concentration of the active ingredient, petasins, remained above the NLT required percentage and within 90% of the initial reported petasin concentration at the end of both testing periods. Although petasin is known to spontaneously transform into isopetasin (Kleeberg-Hartmann et al., 2021), the HPLC method used in this study measures both petasins and isopetasin. Notably, both forms exhibit therapeutic effects (Kleeberg-Hartmann et al., 2021; Borlak et al., 2022). While petasins are prone to degradation by oxidation, hydrolysis, light, and heat, the formulation developed in this study effectively stabilized the compound, preventing degradation for up to 24 months. Additionally, average fill weight, capsule weight variation, identification tests, and rupture time all met the acceptance criteria. Microbial testing for aerobic bacteria, yeasts, and molds also remained within acceptable limits. Based on the results of these stability studies, the shelf life of the product is concluded to be 24 months.
Table 6
Real-time stability data for a single batch of the softgel capsules.
Table 7
Accelerated stability data for a single batch of the finished softgel capsules.
4. CONCLUSION
The present study demonstrated the formulation of softgel capsules containing butterbur, a validated method for the analysis of petasins—the active sesquiterpene ingredients in butterbur—and the 2-year stability of the finished product. This study represents a method of choice for the analysis and formulation of a butterbur-containing herbal product.
Author Contributions
K.A.M. and M.S. contributed to the study conception, analysis, and data curation. K.Z.M. performed part of the statistical analysis and drafted the original manuscript. K.A.M. reviewed the manuscript. All authors contributed to the manuscript revision and read and approved the submitted version.