Introduction
Diabetes mellitus (DM) is major contributor of morbidity and deaths globally. It is identified by chronic hyperglycemia may be occurring due to numerous etiologies. Reports have suggested that, chronic hyperglycemia leads to the disturbances in carbohydrate, protein as well as fat metabolism which could occurs due to insulin deficiency (Punthakee, Goldenberg, & Katz, 2018; Reddy & Lokesh, 1994; Revathy & Abdullah, 2017). Hyperglycemia also activates numerous metabolic pathways like, advanced glycation end products formation (AGE) (Ryle & Donaghy, 1995), hexosamine biosynthesis (Brownlee, 2005), polyol (Edwards, Vincent, Cheng, & Feldman, 2008), and protein kinase C (PKC) pathway overexpression (Leh, 2022). Hyperglycemia can produce mitochondrial reactive oxygen species (ROS) through all the above pathways (Reddemma, Yerra, Areti, Komirishetty, & Kumar, 2014).
Diabetic neuropathy (DN) is the secondary complication caused due to DM and the most prevalent types of neuropathy (Dobretsov, Backonja, Romanovsky, Stimers, & R, 2011), persistent hyperglycemia causes DN in most of the patients (Balducci et al., 2014). Many investigations described association of oxidative damage and thereby ROS in event of DN (Balducci et al., 2014; Safakhah et al., 2017; White et al., 2014).
Oxidative stress (OS) correlated with several abnormalities, including decreased neuronal support, abnormal axonal outgrowth, damage to peroxynitrite DNA, poly-ADP ribose polymerase (PARP) overexpression etc. This suggests that OS may be the common pathway in diabetes that causes damage to the nervous system (Schmeichel & Schmelzer, 2003; Vincent, Kato, Mclean, Soules, & Feldman, 2009; Zherebitskaya, Akude, Smith, & Fernyhough, 2009). Recent animal studies, showed increased degree of lipid peroxidation in addition to decreased SOD, CAT and GSH indicating that involvement of OS in STZ-induced DN (Bansode et al., 2018; Matough, Budin, Hamid, Alwahaibi, & Mohamed, 2012).
Medicinal plant contains a number of antioxidant phytochemicals, such as carotenoids, flavonoids, polyphenols, tannins, saponins, vitamins and α-lipoic acid (Rajendrian, 2017). Among other carotenoids, Lycopene is a fat-soluble compound, generally found in tomatoes and display various beneficial properties such as antioxidant (Jumah, Shahid, Jamal, & Bismilla, 2022), anti-obesity (Zhu et al., 2020), analgesic and anti-inflammatory (Radmehr et al., 2016) and immunomodulation (Puah, Juriyati, & Ali, 2021). It has potential to capture peroxyl radicals along with quenching of singlet oxygen (Bayramoglu, Bayramoglu, Uyanoglu, & Colak, 2015).
Coenzyme Q10 (CoQ10), popularly referred as CoQ10 or ubiquinone, is a lipophilic molecule. It is present in the mitochondria of the majority of eukaryotic cells (Hodgson, Watts, Playford, Burke, & Croft, 2002). CoQ10 has antioxidant properties and is essential for mitochondrial oxidative phosphorylation. It is a potent antioxidant, and shield cells from oxidative damage (Rosenfeldt, Haas, Krum, & Hadj, 2007).
Reports suggested that lycopene interact with vitamin C, E, β-carotene and lutein, thereby increases its free radicals scavenging activity in the lipoprotein particle (Leh, 2022; Pansare, Upasani, Upaganlawar, Sonawane, & Patil, 2021; Upaganlawar, Gandhi, & Balraman, 2010), no study have been reported the effects of combination of lycopene and CoQ10 in neuropathic pain. Based on the available findings, this reaserch was outlined to described the effect of lycopene and CoQ10 alone or in consolidation in peripheral diabetic neuropathy in animals.
Materials and Methods
Drug and chemicals
Lycopene purchased from Universal Industries, Sinnar (Nashik). CoQ10 was received from Zydus, Ahmedabad (India). STZ was procured from Sigma Aldrich (USA). All chemicals and reagents required were of analytical grade.
Experimental Animals
Adult male wistar rats (200-250 g) were divided in different groups. The rats were acquired from Wockhardt Ltd. Aurangabad and housed in the cages with bedding material. The rats were housed in standard laboratory condition according to CPCSEA. The research methods and protocol used were authorized by the IAEC of the institute, (SSDJ/IAEC/2022-23).
Neuropathic pain (NP Induction:
Adult male albino rats were injected subcutaneously by STZ, 55mg/kg once. After 72 hours blood sugar was assessed by glucometer (Roche, Germany). Rats whose blood sugar level was found to be more than 250mg/dL were selected for the studies. During four weeks, the diabetic rats were monitored for the onset of neuropathy. Neuropathy and glucose level were assessed every week by von frey filaments and glucometer.
Experimental Protocol
Diabetic rats were classified into five different groups of six rats each. Treatment started from day one and continue for four weeks.
Group l: control rats that received vehicle for four weeks, Group ll: rats injected with STZ (55mg/kg/s.c.) in 0.1M buffer (citrate pH 4.5) and consider as DN rats, Group lll: DN rats receiving lycopene (5mg/kg/p.o./day) for 4 weeks, Group lV: DN rats received CoQ10 (10 mg/kg/p.o./day) from week 1 to 4, Group V: DN rats administered with lycopene (5mg/kg/p.o./day) and CoQ10 (10mg/kg/p.o./day) daily for 4 weeks. Dose of STZ (Mahajan, Upasani, Upaganlawar, & Gulecha, 2020), Lycopene (Pansare et al., 2021) and CoQ10 (Mahajan et al., 2020) was selected as given in the literature. All the group were assessed after every week and at the end of 4th week for behavioral parameters. At last, animals were sacrificed and the markers of oxidative stress were assessed.
Assessment of DN
All the behavioral parameters were assessed every week using von frey hair (mechanical allodynia) filament and it was expressed as 50% gm threshold (Chaplan, Bach, Pogrel, Chung, & Yaksh, 1994), Mechanical hyperalgesia was evaluated by randall-sellitto analgesiometer and it was expressed as % compression break (% CBK) (Nogueira, Castro, Mancuso, & Navarro, 2012), Heat/thermal hyperalgesia was carried out using eddy’s hot plate analgesiometer and taken as % maximal possible effect (%MPE) (Yalcin, Charlet, Mercier, Barrot, & Poisbeau, 2009) and walking track analysis was expressed as sciatic functional index (SFI) (Mehta, Nerkar, & Banerjee, 2017). The glucose level was determined using digital glucometer. For biochemical analysis the rats were sacrificed by high dose of sodium pentobarbital. C- Reactive Protein level (CRP) was measured in blood serum. Sciatic nerve of rats were isolated and homogenate was prepared as suggested by (Pawar, Upaganlawar, & Upasani, 2021).
Oxidative stress markers:
Assay of Lipid Peroxidation (LPO) (Slater & Sawyer, 1971)
Equal volume of supernatant of sciatic nerve homogenate was mixed with 10 % w/v trichloroacetic acid (TCA). The contents were placed in ice-cold condition for 15 min. The precipitate developed was move apart by centrifugation. Then 2.0 ml of supernatant was added in thiobarbituric acid (TBA). The resultant mixture was heated and cooled immediately. The pink chromogen thus obtained was measured at 532 nm against blank. The standard MDA were taken in different concentration (0-23 nM) for obtaining standard curve.
Measurement of Tissue Nitrite Level (NO) (Guevara et al., 1998)
1ml of homogenate added into Griess reagent and stored for 15 min (37 °C). Measured absorbance of above solution at 540 nm against blank. Sodium nitrite were used as standard. The concentration of NO present in mixture was found by standard curve.
Assay of Superoxide dismutase (SOD) Mishra and Fridovich (1972)
Sciatic nerve homogenate was diluted with equal volume of double distilled water. To the resultant mixture, 0.25 ml of chilled ethanol and chloroform were added. The solution was mixed the thoroughly and centrifuged at 2500 rpm. The supernatant collected was mixed equally with carbonate buffer and EDTA solution. Reaction was initiated by adding 0.4 ml epinephrine and absorbance was noted at 480 nm against blank. The calibration curve was obtained by 10-125 units of SOD.
Assay of Glutathione (GSH) (Moron & Depierre, 1979)
2.0 ml of supernatant and 20 % TCA were mixed together. The resultant precipitate was separated by centrifugation. Then 0.25 ml of supernatant was added into 2.0 ml 5, 5’-dithiobis 2-nitrobenzoic acid (DTNB) reagent and phosphate buffer was added to make the final volume of 3.0 ml. The yellow color obtained was measured at 412 nm. 10-50 µg concentration of standard glutathione was taken for the calibration curve.
Assay of Catalase (CAT) (Luck, 1971)
2.0 ml of nerve homogenate was mixed with hydrogen peroxide (1.0 ml) to start the reaction. The blank was prepared by adding homogenate in 1.0 ml of phosphate buffer (50 mM, pH 7.0) and the decrease in the absorbance was determined at 240 nm.
Statistical Analysis
The statistical significance was determined using Graph Pad Prism (version 5.01). ANOVA then Dunnett’s test was used for evaluation. The values were indicated as mean and SEM (n=6). The p < 0.05 was statistically significant in all the tested parameters.
Results
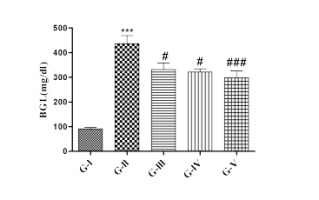
Effect of lycopene and/or CoQ10 on fasting blood glucose level (BGL)
As shown in Figure 1, 72 hrs post- STZ injection, BGL was determined. DN rats showed significantly (p<0.001) elevated blood glucose than control rats. The elevation in BGL was continued till the end of 4th week in diabetic rats as compared to control group. DN rats receiving lycopene and CoQ10 alone showed significant (p<0.05) decreased and their combination showed more significant (p<0.001) reduction in BGL than DN rats.
Figure 1
Effect of lycopene, CoQ10 and their combination on blood glucose level (4th week) in STZ induced DN rats.
Values are expressed as mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett ‘t’ test. *P<0.05, **P<0.01, ***P<0.001 compared to control and #P<0.05, ##P<0.01, ###P<0.001 compared to DN.
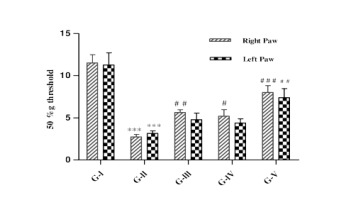
Effect of lycopene and/or CoQ10 on mechanical allodynia
Mechanical allodynia was evaluated using von Frey filaments and expressed as 50% gm threshold. As shown in Figure 2, 50% gm threshold of both the hind paws of DN rats was decreased significantly (p<0.001) than the control animals. DN rats receiving lycopene alone displayed significant (p<0.01) elevation in 50% gm threshold in right hind paws and left hind paws showed not significant results as compared to DN rats. DN rats receiving CoQ10 alone showed significant (p<0.05) increased in 50% gm threshold in right hind paws and left hind paws showed not significant results when compared with DN rats. The DN rats administered with combination of lycopene and CoQ10 showed more significant (p<0.001) effect in right hind paws and left hind paws showed significant (p<0.01) effect in increasing 50% gm threshold when compared to DN rats.
Figure 2
Effect of lycopene, CoQ10 and their combination on mechanical allodynia using von frey hair filament test in STZ induced DN rats.
Values are expressed as mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett ‘t’ test. *P<0.05, **P<0.01, ***P<0.001 compared to control and #P<0.05, ##P<0.01, ###P<0.001 compared to DN.
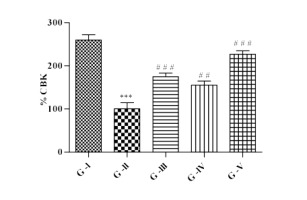
Effect of lycopene and/or CoQ10 on mechanical hyperalgesia
Mechanical hyperalgesia was carried out in left hind paw of DN rats using randall sellitto analgesiometer and expressed in % CBK. Figure 3, indicates that there was significant (p<0.001) reduced in % CBK in DN rats than control rats. DN rats administered with lycopene alone showed significant (p<0.001) elevation in % CBK and DN rats administered with CoQ10 showed significant increase (p<0.01) in % CBK as than DN rats. The administration of lycopene and CoQ10 in combination to DN rats displayed significant (p<0.001) effect in increased % CBK than DN rats.
Figure 3
Effect of lycopene, CoQ10 and their combination on mechanical hyperalgesia using Randall Sellitto analgesiometer in STZ induced DN rats.
Values are expressed as mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett ‘t’ test. *P<0.05, **P<0.01, ***P<0.001 compared to control and #P<0.05, ##P<0.01, ###P<0.001 compared to DN.
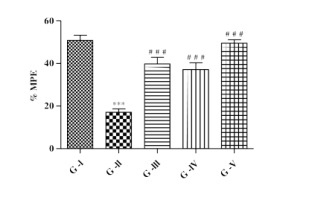
Effect of lycopene and/or CoQ10 on heat/thermal hyperalgesia
Eddy’s hot plate was used to assess thermal hyperalgesia in DN rats and expressed in % MPE. It was observed that, in DN rats there was a significant (p<0.001) decrease in % MPE in comparison to control group. Animals with DN that received lycopene, CoQ10 or their combination showed significant (p<0.001) raised in % MPE when compared with DN rats (Figure 4).
Figure 4
Effect of lycopene, CoQ10 and their combination on heat/thermal hyperalgesia using Eddy’s hot plate test in STZ induced DN rats.
Values are expressed as mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett ‘t’ test. *P<0.05, **P<0.01, ***P<0.001 compared to control and #P<0.05, ##P<0.01, ###P<0.001 compared to DN.
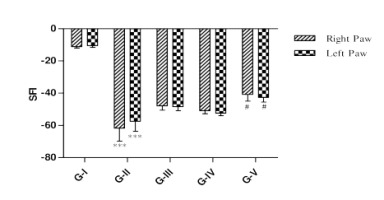
Effect of lycopene and/or CoQ10 on walking track analysis
As shown in Figure 5, the SFI was observed in both right and left hind paw in all the groups. DN rats exhibited significant (p<0.001) elevation in SFI of both the paw when compared to normal rats. DN rats treated with lycopene and CoQ10 alone decreased the SFI of both the paw as compared to DN rats, but it was not found to be significant. DN rats administered with combination of lycopene and CoQ10 displayed a significant (p<0.05) decreased in SFI of both the paw compared to DN . as well as better effects than lycopene and CoQ10 alone treatment.
Figure 5
Effect of lycopene, CoQ10 and their combination on walking track analysis in STZ induced DN rats.
Values are expressed as mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett ‘t’ test. *P<0.05, **P<0.01, ***P<0.001 compared to control and #P<0.05, ##P<0.01, ###P<0.001 compared to DN.
Effect of lycopene and/or CoQ10 on biomarkers of oxidative stress
It was observed that, that the concentration of LPO (Figure 6a) in homogenate of sciatic nerve of DN rats was elevated significantly (p<0.001) when compared to control animals. DN rats administered with lycopene, CoQ10 or concomitant of lycopene and CoQ10 showed significant reduction (p<0.001) in LPO concentration after 4-week treatment when compared to DN rats.
Figure 6
Effect of lycopene, CoQ10 and their combination on Biomarkers of oxidative stress 6 (a) Lipid Peroxide (LPO) and 6 (b) Nitric Oxide (NO) in STZ induced DN rats.
Values are expressed as mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett ‘t’ test. *P<0.05, **P<0.01, ***P<0.001 compared to control and #P<0.05, ##P<0.01, ###P<0.001 compared to DN.
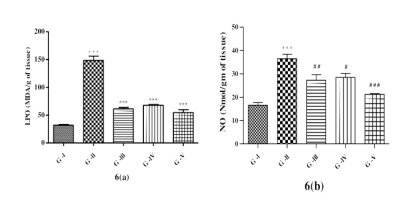
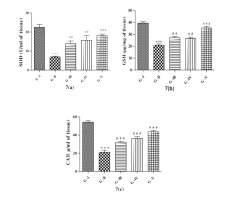
Figure 7
Effect of lycopene, CoQ10 and their combination on Biomarkers of oxidative stress 7 (a) Superoxide Dismutase (SOD), 7 (b) Reduced Glutathione (GSH) and 7 (c) Catalase (CAT) levels in STZ induced DN rats.
Values are expressed as mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett ‘t’ test. *P<0.05, **P<0.01, ***P<0.001 compared to control and #P<0.05, ##P<0.01, ###P<0.001 compared to DN.
NO (Figure 6b) in the homogenate of DN rats found to be raised significantly (p<0.001) as compared to the control. DN rats received lycopene showed significant (p<0.01) declined in NO levels when compared with DN rats. DN rats administered with CoQ10 showed decreased significantly (p<0.05) in NO levels when compared with DN rats. DN rats administered with combination of both antioxidants exhibited significant (p<0.001) decreased in NO levels when compared to DN group.
Fig.7 showed, alteration in oxidative stress markers of in DN rats. The concentration of SOD (Figure 7a), GSH (Figure 7b) and CAT (Figure 7c) in the sciatic nerve homogenate of DN rats was significantly (p<0.001) decreased correlated with control rats, suggesting the involvement of OS in DN. Treatment with lycopene and CoQ10 alone significantly (p<0.01) ameliorated the activity of biomarkers of OS (SOD and GSH). Therapy with the combination of both the antioxidant significantly (p<0.001) elevated SOD and GSH levels, but in case of CAT after dose with lycopene and CoQ10 alone and combo of both antioxidants displayed significant raised in levels of CAT when correlated to DN.
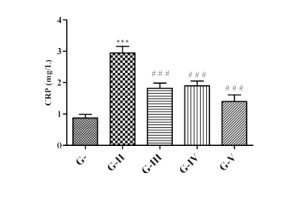
Effect of lycopene, CoQ10 and their combination on CRP level
The present research showed that the concentration of CRP (Figure 8) was raised significantly (p<0.001) when correlated with control, indicating that there was inflammation due to DM. After the treatment with lycopene, CoQ10 and their combination significantly (p<0.001) reduced the CRP level than DN group.
Discussion
DM is responsible for gluconeogenesis (Kaeidi et al., 2011). Long term hyperglycemia is considered as the important cause of all complications associated to DM, including DN. Studies showed that, raised blood glucose plays crucial role in the development OS (Mahajan, Upasani, & Upaganlawar, 2019). DN develops due to uncontrolled management of hyperglycemia. Symptoms of DN includes pain, slow nerve conduction and tingling in the extremities (Sezer, Usta, Kocak, & Yagci, 2011).
In the present research, rats were rendered diabetic by the single subcutaneous dose of STZ to induce DN. It also causes degeneration of islets of beta cells in langerhans and activation of protein kinase-C which leads to the production of ROS and thereby causes nerve damage and neuropathy (Warokar & Sawant, 2021). STZ administered rats showed elevated level of blood glucose than control group. Raised in blood glucose was reported in DM induced by STZ to study diabetes and its complications (Pansare et al., 2021).
There are many approaches available to overcome DN. The present investigation is mainly focused on antioxidant therapy. Antioxidant are compound which decreases OS by scavenging free radicals. Many antioxidants are available with a potential to reduced autoimmune disease, free radical formation and protects cells against oxidative damage. In our investigation, it was observed that rats administered with lycopene, CoQ10 and their combination showed reduction in elevated blood glucose level. These findings are harmonious with the previous literatures (Mahajan et al., 2020; Pansare et al., 2021).
Behavioral indicators that identify nociceptor functioning in DN rats were used in this investigation. This includes mechanical allodynia, heat or thermal hyperalgesia, mechanical hyperalgesia, and walking track analysis (Mehta et al., 2017). Evaluation of behavioural results to outermost incentives in DN rats gives beneficial information about the process of atypical sensation or pain related with DM (Muller, Ryals, Feldman, & Wright, 2008). In this research, the pain threshold in DN rats was found to be significantly decreased after testing with von frey hair filaments, randall sellitto and hot plate analgesiometer. This reduction in pain threshold might be due to damage to sensory and motor fibers (Courteix, Bardin, Chantelauze, Lavarenne, & Eschalier, 1994). Previous literature also showed the same effects on pain threshold in STZ induced DN (Jain, Bansal, Dalvi, Upganlawar, & Somani, 2014; Sanklecha, Upaganlawar, & Upasani, 2017; Warokar & Sawant, 2021). Rats administered with lycopene, CoQ10 and their combination displayed significant elevation in pain threshold compared to DN rats.
Walking track analysis is used for the assessment of functional condition of the sciatic nerve at the time of regeneration. Coordinated function like cortical integration, sensory input, and motor response are required for proper walking. (Kunkel-Bagden & Bregman, 1990).
The present examination indicates that, the sciatic functional index (SFI) of DN rats treated with lycopene and CoQ10 alone reduced the elevated SFI whereas rats treated with combination of both lycopene and CoQ10 reduces the SFI more significantly than alone antioxidants.
STZ administered rats showed LPO and NO levels in sciatic nerve was found to be significantly rised whereas SOD, GSH and CAT levels was significantly declined as compared to normal.
STZ administration causes the free radicals formation and thereby oxidative damage in DN, which may affect the activities of endogenous antioxidant markers. Biomarkers of oxidative stress such as LPO, NO, SOD, GSH and CAT was examined in the present study. Rised in LPO is main indicators of long-term diabetes. Lipid peroxidation may result from free radical elevation in cells. Rise in LPO causes damage to islet cells and thereby responsible for hyperglycemia (Leh, 2022).
NO level was found to be elevated in DN group. Rised level of NO may be due to stimulation of inos and greater serum glucose level accountable for enhancing NO levels by activation of endothelial cells. NO concentration in combination group was significantly reduced as compare to DN. In previous study it was noted that NO level get elevated in DN rats (Bhokare & Upaganlawar, 2016).
SOD is most broadly studied antioxidant enzyme. Furthermore, plasma, lymph, ascites, and cerebrospinal fluid all contain extracellular SOD. It function as first line defence against ROS, by converting O2•− into H2O2 (Apel & Hirt, 2004). Superoxide dismutase play an essential function in oxidative damage by triggering quick dismutation of O2•− species, thus lowering the risk of •OH production in reaction that are metal-catalyzed (Bowler, Montagu, & Inzé, 1992). According to reports, Glutathione (GSH) is a crucial antioxidant, their deficiency elevates oxidative stress (Ismail & Murat, 2013; Warokar & Sawant, 2021). GSH aids in defencing cells from various harmful stimuli, such as free radicals produced by oxygen. CAT depletes H2O2 and guards against extremely reactive radicals in cell.
Administration with lycopene and CoQ10 alone significantly reduced the LPO and NO levels. However, their combination more significantly decreased the elevated levels of LPO and NO. The concentration of oxidative stress biomarkers i.e., SOD, GSH and CAT was significantly reduced in DN rats whereas lycopene, CoQ10 and their combination treatment significantly increases the level of SOD, GSH and CAT compared to DN rats.
Long standing hyperglycemia causes development of ROS which activates various hemodynamic pathways that promotes inflammation and tissue fibrosis. The increased inflammation in DN rats is evaluated by measuring CRP level. It was found that lycopene and CoQ10 causes hypoglycemia and inactivation of hemodynamic pathways which decreased the CRP level.
Studies have demonstrated that antioxidants function synergistically and more productively when they are used together despite of their different property. Reports suggested lycopene work synergistically with various antioxidant such as α-tocopherol, lutein, vitamin C, and β-carotene (Leh, 2022; Upaganlawar et al., 2010). It was revealed that the coefficient effect of lycopene and Vit. E is associated with vit. E regeneration from α- tocopheroxyl radical by lycopene (Upaganlawar et al., 2010). Another study reported that CoQ10 when given in combination with N-acetylcysteine also show synergistic effect due to their antioxidant property (Mahajan et al., 2020). So, this could be the reason for better activity of Lycopene and CoQ10 combination in the DN.
Conclusion
This study revealed that, treatment with lycopene and CoQ10 for 4 weeks in DN showed significant recovery of behavioral and biochemical parameters. The combination of lycopene and CoQ10 significantly reversed hyperalgesia and allodynia, reduced the development of oxidative stress in DN rats than alone. This combination work together as strong antioxidant and thereby protects all the alteration observed during DN.