1. INTRODUCTION
As a universal biological phenomenon, aging leads to an inevitable decline in organ function over time, primarily due to accumulated damage from various environmental and physiological stressors (Bajpai et al., 2021; Guo et al., 2022). Both intrinsic and extrinsic factors contribute to the skin aging process (Hussein et al., 2024). Intrinsic factors are mainly related to chronological, genetic, and cellular metabolism, while extrinsic factors arise from environmental elements and lifestyle, such as air pollution, smoking, nutrition, and ultraviolet (UV) radiation (Hussein et al., 2024; Wong & Chew, 2021). UV radiation, a prevalent environmental factor, gradually surpasses the protective capacity of melanin within the outermost layer of the skin, promoting rapid progression of skin damage in aging (Brenner & Hearing, 2008; Gromkowska-Kępka et al., 2021). People living near the equator have higher melanin levels in their epidermis, providing better protection against UV radiation compared to those in higher latitudes; however, a study suggests that this melanin is insufficient to fully shield the skin from extended contact with intense UV rays (Bae et al., 2016). While aging signs like wrinkles, sagging, and thinning skin usually appear in the mid-third decade of life, a study suggests that younger Asians are increasingly aware of early signs of aging, such as dull skin, uneven pigmentation, dryness, fine lines, and decreased elasticity (Anwar et al., 2022).
The oxidative stress hypothesis of aging proposes that the progressive loss of function with age stems from accumulated damage triggered by oxidative and nitrosative species, positioning oxidative stress as a pivotal element in aging and a fundamental mechanism underlying age-associated diseases (Iakovou & Kourti, 2022; Liguori et al., 2018). The degradation of the extracellular matrix (ECM) due to heightened collagenase, hyaluronidase (HAase), and elastase activities results in reduced skin elasticity and firmness (Garg et al., 2017; Jiratchayamaethasakul et al., 2020; Vinardell et al., 2024). Inhibition of these enzymes (collagenase, HAase, and elastase) has been associated with reduced signs of aging, emphasizing the importance of targeting them in antiaging strategies (Das et al., 2024; He et al., 2024).
At the genetic level, aging-related changes in gene expression, affecting ECM homeostasis, inflammation, and apoptosis, can worsen the aging process (Freitas-Rodríguez et al., 2017). Reactive oxygen metabolites and oxidative disruption contribute to genomic damage, initiating the upregulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) as well as the production of intercellular signaling proteins, including interleukin-6 (IL-6), ultimately disturbing fibroblast and keratinocyte homeostasis (Hong et al., 2024; Reeve et al., 2009). The inflammatory process then increases activator protein-1 (AP-1), which subsequently activates matrix metalloproteinase-1 (MMP)-1, leading to type I collagen catabolism (Freitas-Rodríguez et al., 2017). Unregulated buildup of reactive oxygen metabolites and genomic instability can trigger the intrinsic apoptotic pathway via caspase-3, causing fibroblast and damaged cell death, which worsens tissue degradation and impairs skin regeneration (Panneer Selvam et al., 2018; Shalini et al., 2015).
Synthetic chemical antiaging products have drawn substantial attention in the cosmetic industry; however, they are often accompanied by environmental and health effects (Wirtu, 2024). Among the most commonly used synthetic antiaging agents are alpha hydroxy acids (AHAs) and retinoids, which are known for their ability to promote skin refinement, decrease facial folds, and boost the overall appearance of delicate lines and rough patches (Milosheska & Roškar, 2022; Moghimipour, 2012). However, although these compounds are effective, they can also cause skin irritation (Almeman, 2024; Milosheska & Roškar, 2022). Therefore, natural plant-derived antioxidants are needed (Liu, 2022).
The potential role of beetroot (Beta vulgaris L.) as an antiaging agent has attracted attention due to its rich composition of bioactive compounds such as betalains, phenolics (gallic acid), and flavonoids (quercetin and kaempferol) (Punia Bangar et al., 2022). The phytochemical compounds in beetroot are important contributing factors in combating oxidative stress, as an ultimate event of skin aging (Huma et al., 2020). Various parts of beetroot, such as leaves, bulb, stem, powder, and skin extracts, have shown promising results as antioxidants, have anti-inflammatory properties, and even prevent cancer (Clifford et al., 2015). However, most research has focused on the beetroot bulb, leaves, or juice extract, while the potential antiaging benefits of beetroot peel extract (BPE) remain largely unexplored. Furthermore, limited studies have evaluated its effects on skin-aging markers at the molecular level, particularly in UVB-induced fibroblast models.
This study aims to evaluate the potential role of BPE as an antiaging agent by assessing its free radical scavenging activity and measuring its effects on protein levels and enzyme inhibition. Furthermore, the cytotoxicity and gene expression analyses were conducted in UVB-induced human fibroblast cells to determine its safety and efficacy as an antiaging agent. By assessing biochemical and cellular responses, this study comprehensively evaluates the potential of BPE in combating oxidative stress, inflammation, and collagen degradation—key hallmarks of skin aging. These findings could contribute to the development of BPE-based natural antiaging formulations, offering an effective and safer alternative to synthetic antiaging agents.
2. METHODS
2.1. Extract preparation
Manufactured by PT. Fathonah Amanah Shiduq Tabligh (FAST), located in Depok 16453, West Java, Indonesia, BPE adheres to Good Manufacturing Practices (GMP) and corresponds to CoA Batch 001AK68A118. The beetroot peel was extracted by soaking it in 70% ethanol, followed by filtration. The resulting filtrate was evaporated at 40 °C until a paste was formed, after which lactose was added as an excipient (Widowati et al., 2023a).
2.2. DPPH scavenging assay
A reaction mixture, consisting of 50 µL of BPE (6.25–200 µg/mL) and 200 µL of 0.077 mM DPPH in methanol, was incubated at ambient temperature for 30 min. The optical density at 517 nm was determined using a microplate reader (Girsang et al., 2020; Widowati et al., 2017). Radical scavenging efficiency was computed based on the following mathematical expression.
Five activity levels were established: very strong (<10 µg/mL), strong (10–50 µg/mL), weak (50–100 µg/mL), very weak (100–250 µg/mL), and inactive (>250 µg/mL) (Reviana et al., 2021; To’bungan et al., 2024).
2.3. Elastase inhibition assay
A microplate-based incubation was conducted using 10 µL of BPE (6.25–200 µg/mL) and 5 µL of porcine pancreas elastase (Sigma-Aldrich, St. Louis, MO, USA; Cat. No. 45124, 0.5 mU/mL in cold distilled water). The mixture was incubated at 25 °C for 15 min with 125 µL of Tris buffer (100 mM, pH 8). Afterward, 10 µL of Suc-Ala-Ala-Ala-pNA (2 mg/mL in Tris buffer) supplied by Sigma-Aldrich (St. Louis, MO, USA), catalog reference 54760, was added, and the reaction was allowed to proceed for another 15 min at 25 °C. The reaction was monitored by measuring absorbance at 410 nm using a microplate reader (Widowati et al., 2017). Inhibition activity was calculated using the following equation:
2.4. Cell culture
This study used the ATCC® CRL-2522 human fibroblast (BJ) cell lines from American Type Culture Collection (Manassas, VA, USA). Cells were procured from Aretha Medika Utama. Culturing was carried out in a complete minimum essential medium (MEM) supplemented with 10% FBS at 37 °C with 5% CO (Girsang et al., 2019; Widowati et al., 2024). The culture medium was refreshed every 2–3 days to promote healthy cell proliferation. Cell morphology was examined under an inverted microscope to track any changes in structure, confluency, and adherence (Olympus, CKX41, Olympus Corporation, Tokyo, Japan).
2.5. Cytotoxic assay
Cytotoxicity tests were carried out according to Widowati et al. (2021) with modification. Cells (1 × 104 per well) were plated in 96-well plates and maintained at 37 °C, 5% CO2 for 24 h to ensure proper adhesion. Following this incubation, the existing medium was discarded and substituted with 180 µL of MEM (2% FBS), to which 20 µL of BPE at graded concentrations (0.82, 1.63, 3.13, 6.25, 12.5, 25, and 50%) was introduced. Cells remained incubated for another 24 h under unchanged conditions to assess the effects of BPE treatment. On the following day, 20 µL of WST-8 sourced from Elabscience (E-CK-A362; Elabscience Biotechnology Inc., Houston, TX, USA) was dispensed into every well and incubated for 3 h at 37 °C to analyze cell viability and assess potential inhibitory effects of the treatment (Zou et al., 2019). The treatment groups comprised negative control (NC; BJ cells without treatment), DMSO control (DMSO; NC + DMSO 1%), and BPE of various concentrations. A Thermo Scientific Multiskan™ FC spectrophotometer (1510-00778C; Thermo Fisher Scientific, Waltham, MA, USA) was employed to determine the absorbance at 450 nm. The three best BPE concentrations were selected for further testing.
2.6. Bradford assay
Total protein was assayed from a conditioned medium of UV-induced BJ cells treated using the Bradford assay. Six treatment groups were established: (1) Negative Control (NC) with untreated BJ cells; (2) Positive Control (PC) with UV-induced BJ cells without additional treatment; (3) DMSO Control (PC + 1% DMSO); and (4) three experimental groups treated with BPE at increasing concentrations: BPE 3.91 (PC + 3.91 µg/mL), BPE 15.63 (PC + 15.63 µg/mL), and BPE 62.5 (PC + 62.5 µg/mL). UV induction was carried out using UVB light for 75 min (300 J/cm2) before the cells were treated (Girsang et al., 2023). A standard protein calibration curve was developed using BSA (Sigma-Aldrich, A9576, MO, USA), dissolved at 2 mg in 1000 μL ddH2O and serially diluted. Following this, 20 μL of both standards and sample protein solutions were aliquoted into a 96-well plate, and 200 μL of Quick Start™ Dye Reagent 1X (Bio-Rad, 5000205, CA, USA) was subsequently added. A 5-minute room temperature incubation was followed by absorbance measurement at 595 nm using a microplate reader (Lister et al., 2022).
2.7. Protein expression analysis: colorimetry and ELISA test
The total antioxidant levels were analyzed using the Colorimetric Total Antioxidant Capacity (T-AOC) Assay Kit (Elabscience, E-BC-K136-S; Elabscience Biotechnology Inc., Houston, TX, USA). A specific ELISA kit was employed to determine HAase protein levels, ensuring accurate quantification (Elabscience, E-EL-H2201; Elabscience Biotechnology Inc., Houston, TX, USA). The procedure was completed per the manufacturer’s recommendations (Widowati et al., 2024).
2.8. Gene expression analysis (qRT-PCR): IL-6, Casp-3, MMP-1, and COL1A1
The expression levels of IL-6, Casp-3, MMP-1, and COL1A1 genes were measured from treated pellet cells using real-time PCR (qRT-PCR). Using the Direct-zol™ RNA Miniprep Plus Kit (Zymo Research, R2073), total RNA was extracted, following the standardized procedure outlined by the manufacturer. The concentration and purity of the isolated RNA are summarized in Table 1. For complementary DNA (cDNA) synthesis, the Sensi-FAST cDNA synthesis kit (Meridian, 65053) was used according to the manufacturer’s protocol. The synthesized cDNA was then used as a template for qRT-PCR analysis. The AriaMx 3000 Real-Time PCR System (Agilent, G8830A) and SensiFast™ SYBR® No-ROX (Meridian, BIO-98005) were utilized for real-time PCR quantification of target genes. The procedure followed the manufacturer’s protocol (Widowati et al., 2023b). The primer sequence can be seen in Table 2. GAPDH was employed as an endogenous control for data normalization. The specificity of amplification was confirmed by melting curve analysis. The 2−ΔΔCt method was applied to quantify relative gene expression levels. The negative control group was used as the reference (baseline) for expression comparison. The fold expression values indicate upregulation (>1) or downregulation (<1) of target genes in response to BPE treatment.
Table 1
Quantification and purity assessment of RNA.
Table 2
Primer sequences selected for this experiment.
2.9. Statistical analysis
Mean ± SD is used to represent all values. Statistical processing was executed using SPSS software (version 23.0). Data originated from three repetitions for the cytotoxicity assay and four for colorimetry, ELISA, and qRT-PCR. Tukey’s HSD post hoc test was applied following one-way ANOVA for normal and homogeneous data. Nonhomogeneous data were analyzed using Dunnett T3, while nonparametric analysis was conducted using the Kruskal–Wallis test with the Mann–Whitney U test for post hoc comparisons. The cutoff for significance was set at p ≤ 0.05.
3. RESULTS
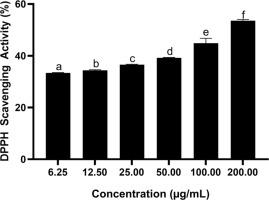
3.1. Effect of BPE on DPPH radical neutralization activity
Results confirmed that BPE exhibited stronger DPPH radical neutralization activity at higher concentrations. BPE at 200 μg/mL was the most effective concentration (Figure 1). The IC50 value representing DPPH radical neutralization capacity is listed in Table 3.
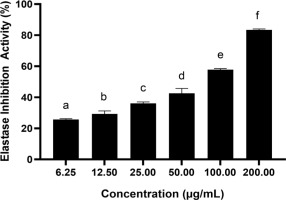
3.2. Effect of BPE on elastase inhibitory activity
Figure 2 shows a notable enhancement in elastase inhibitory activity (p < 0.05) along with the increase in BPE concentration, with the highest activity reaching about 83.45% at a concentration of 200 µg/mL. IC50 values < 200 µg/mL signify an active inhibitory capacity; so, the value 80.14 µg/mL (Table 4) indicates a very active elastase inhibitory activity of BPE.
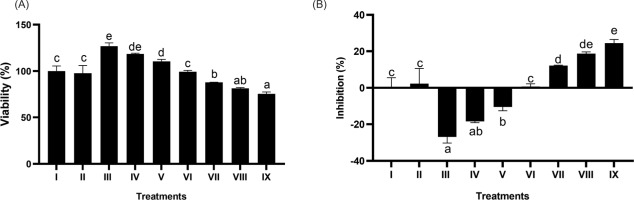
3.3. Effect of BPE on cytotoxicity: viability and inhibition
The outcome of the cytotoxic evaluation is depicted in Figure 3. The dose used in this test is still within a safe limit. BPE at 7.81–62.5 µg/mL is safe, maintaining cell viability at > 90%. Through Probit regression analysis, the IC50 level was established at 1683.455 µg/mL.
Figure 3
Effect of BPE toward viability (A) and inhibition (B) of BJ cells. Distinct codes indicate significant differences (p < 0.05) according to Tukey’s HSD post hoc comparison. I (BJ cells without treatment); II (NC + DMSO 1%); III (NC + BPE 500 μg/mL); IV (NC + BPE 250 μg/mL); V (NC + BPE 125 μg/mL); VI (NC + BPE 62.5 μg/mL); VII (NC + BPE 31.25 μg/mL); VIII (NC + BPE 15.63 μg/mL; IX (NC + BPE 7.81 μg/mL).
3.4. Effect of BPE on total protein levels
The effect of BPE on total protein levels can be seen in Figures 4A and 4B. A notable increase in total protein levels was detected (p < 0.05) in BPE-treated samples compared to PC, with BPE 62.5 µg/mL producing the most substantial enhancement.
Figure 4
Effect of BPE toward total protein content in aging cells model. Protein content (ug/mL) (A) and protein content (mg/mL) (B) I: negative control, untreated cells (NC); II: positive control (PC), UV-induced cells; III: DMSO, PC treated with DMSO1%; IV: PC treated with BPE 3.91 μg/mL; V: PC treated with BPE 15.6 μg/mL; VI: PC treated with BPE 62.5 μg/mL. Distinct codes indicate significant differences (p < 0.05) according to Tukey’s HSD post hoc comparison.

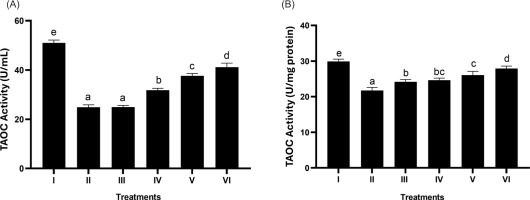
3.5. Effect of BPE on the total antioxidant level (T-AOC)
Based on Figures 5A and 5B, it is observed that BPE can significantly increase T-AOC levels. The most active treatment in increasing T-AOC was shown by BPE 62.5 μg/mL (p < 0.05).
Figure 5
Effect of BPE toward TAOC levels in aging cells model. TAOC in U/mL (A) and TAOC in U/mg protein (B). I: negative control, untreated cells (NC); II: positive control (PC), UV-induced cells; III: DMSO; IV: BPE 3.91 ug/mL; V: BPE 15.6 ug/mL; VI: BPE 62.5 ug/mL. Distinct codes indicate significant differences (p < 0.05) according to Tukey’s HSD post hoc comparison.
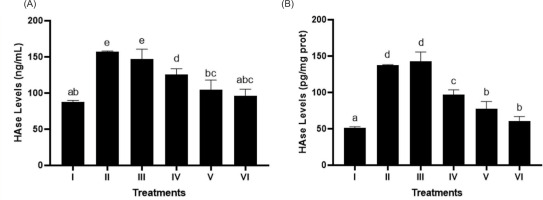
3.6. Effect of BPE on HAase level
In Figures 6A and 6B, BPE treatment notably suppressed HAase levels (p < 0.05) in UV-exposed BJ cells, modeling aging, with BPE 62.5 µg/mL being the most effective concentration.
Figure 6
Effect of BPE toward HAase levels in aging cells model. HAase levels in ng/mL (A) and HAase levels in pg/mg protein (B). I: negative control, untreated cells (NC); II: positive control (PC), UV-induced cells; III: DMSO; IV: BPE 3.91 μg/mL; V: BPE 15.6 μg/mL; VI: BPE 62.5 μg/mL. Different codes indicate significant differences between treatments based on the Mann–Whitney test (p < 0.05).
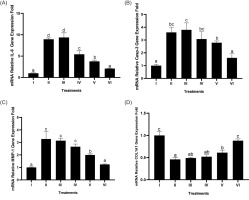
3.7. Effect of BPE on IL-6, Casp-3, MMP-1, and COL1A1 gene expression
As evidenced by the study findings on the regulation of BPE on various genes (IL-6, Casp-3, MMP-1, COL1A1) in BJ cells induced by UV light, the highest BPE concentration gave the best results for each gene used (Figure 7). BPE treatment decreased gene expression (IL-6, Casp-3, and MMP-1), while COL1A1 gene expression increased significantly (p < 0.05). The most effective treatment was BPE 62.5 µg/mL. From these results, it can be concluded that BPE acts as an anti-inflammatory, antiapoptotic, and antiaging agent.
Figure 7
Effect of BPE toward IL-6 (A), Casp-3 (B), MMP-1 (C), and COL1A1 (D) genes expression in aging cells model. I: negative control, untreated cells (NC); II: positive control (PC), UV-induced cells; III: DMSO, PC treated with DMSO1%; IV: PC treated with BPE 3.91 μg/mL; V: PC treated with BPE 15.6 μg/mL; VI: PC treated with BPE 62.5 μg/mL. Different codes indicate significant differences among treatments based on Tukey’s HSD post hoc test (p < 0.05).
4. DISCUSSION
Ultraviolet exposure produces prooxidant molecules, causing a shift in redox equilibrium (Gromkowska-Kępka et al., 2021). The resulting oxidative stress leads to oxidative damage that disrupts transcription factors and signaling pathways such as NF-κB and MAPK, ultimately accelerating skin aging by promoting inflammation and collagen degradation (Hong et al., 2024; Iakovou & Kourti, 2022; Liguori et al., 2018). In this context, antioxidants, especially those derived from natural ingredients, are important in neutralizing ROS and restoring redox balance (Hussen et al., 2025; Liu, 2022).
The potential of BPE as an antioxidant was also validated through in vitro testing in the present research. BPE showed DPPH radical quenching ability that increased with increasing concentration (Figure 1), displaying an observed IC50 of 159.69 µg/mL (Table 3). Based on this, BPE has antioxidant activity in the very weak category. However, BPE showed a significant effect of increasing TAOC levels in BJ aging model cells (p < 0.05) compared to PC (Figures 5A and 5B). From this, it is known that BPE has the potential effects of a fairly effective antioxidant. The betalain content in BPE not only imparts a distinctive red color to beetroot but also exhibits strong free radical scavenging and inflammation-inhibiting responses (Clifford et al., 2015; Huma et al., 2020). Existing research supports the potential application of beetroot peel extract as an antioxidant that reduces oxidative stress, exhibits antimicrobial properties, and enhances cell regeneration (Al-Harbi et al., 2021; John & Monica, 2017). Other studies used different parts of beetroot to assess their cosmetic potential and antioxidant properties as an agent for slowing aging processes (Mohsen et al., 2024; Parwata et al., 2023; Zand et al., 2024). A study has also shown that betanin effectively mitigates epigenetic modifications and UV-induced DNA damage in HaCaT cells (Zand et al., 2024).
The results of the cytotoxicity test (Figure 3) show that BPE 7.81–62.5 µg/mL is a safe concentration range with BJ cell viability > 90% (Widowati et al., 2019). These results indicate that BPE is not toxic to BJ cells, which is also supported by the BPE IC50 value of 1683.455 µg/mL. A high IC50 value suggests that BPE has a low level of toxicity because a much higher concentration is needed to cause damage or death of half of the cells (Damiani et al., 2019). Based on the study results, BPE demonstrates strong antioxidant potential and an appropriate safety profile, making it a strong prospect for incorporation into antiaging formulations.
Photoaging causes an increase in ROS, which then activates the MAPK signaling pathway that induces AP-1, which boosts the expression of two skin-aging enzymes, namely elastase and HAase, which are essential for the breakdown of elastin and hyaluronate (Guo et al., 2022; Nisa et al., 2024). These enzymes must be inhibited to maintain skin moisture and elasticity and stop age-related degradation. BPE significantly inhibited elastase in a dose-dependent manner, reaching 83.45% inhibition at 200 µg/mL BPE (Figure 2). The IC50 value of 80.14 µg/mL (Table 4) is significantly lower than the threshold of 200 µg/mL for active antioxidants, indicating the strong antiaging potential of BPE. In addition, the results also showed that BPE 62.5 µg/mL significantly decreased HAse protein quantification in UV-irradiated BJ cells versus PC (Figures 6A and 6B). These results corroborate previous research that proved that red beetroot has an elastase inhibitory activity and another study that stated betalain-enriched beetroot inhibited hyaluronidase activities associated with gut inflammation (Saad et al., 2023; Winanta et al., 2024). The results of this study correlate with the effects of BPE in significantly increasing total protein levels (Figures 4A and 4B). The escalation of the total protein suggests that the extract might promote enhanced protein synthesis, protect against protein degradation, and support fibroblast activation and skin regeneration, potentially mitigating photoaging.
AP-1 activation also increases MMP-1 expression, causing increased collagen degradation (Freitas-Rodríguez et al., 2017). The loss of collagen, the main protein that provides skin strength and elasticity, triggers the appearance of wrinkles and sagging skin (Bajpai et al., 2021; Guo et al., 2022). Moreover, ROS destabilizes the TGF-β/Smad signaling axis, which is essential for collagen production and maintenance (Liu & Desai, 2015). As a result, there is a decrease in COL1A1 gene expression, which causes inhibition of skin tissue regeneration (Potekaev et al., 2021). At the same time, ROS activates NF-kB which triggers the heightened secretion of inflammatory response modulators such as IL-6 (Reeve et al., 2009). Increased IL-6 causes chronic inflammation, which not only damages skin tissue but also inhibits ECM repair, encompassing collagen and elastin, key proteins necessary for skin strength and structural support (Ansary et al., 2021). In addition to affecting signaling and inflammation pathways, UV exposure also causes DNA damage, such as the formation of pyrimidine dimers that trigger the activation of the Casp-3 enzyme, through the apoptosis pathway (Panneer Selvam et al., 2018; Shalini et al., 2015). According to recent research, controlled apoptosis is essential for maintaining skin homeostasis and avoiding the accumulation of damaged cells, which can accelerate the aging process (Anderton & Alqudah, 2022). Casp-3 activation results in fibroblast cell death, causing UV-induced skin tissue damage, reducing skin regeneration capacity and accelerating the aging process (Anderton & Alqudah, 2022; Shalini et al., 2015).
Beetroot peel extract has been found to negatively regulate the expression of genes like IL-6, Casp-3, and MMP-1, while upregulating the expression of the COL1A1 gene (Figures 7A–7D). The decrease in IL-6 expression suggests that BPE effectively mitigates inflammation. IL-6 is a cytokine associated with chronic inflammation and skin damage, often upregulated in response to oxidative stress, such as that caused by UVB exposure (Reeve et al., 2009). By reducing IL-6 levels, BPE likely creates a less inflammatory environment conducive to tissue repair and regeneration. Similarly, the downregulation of Casp-3 indicates a reduction in apoptosis. Caspase-3 is a fundamental effector in the apoptotic cascade (Panneer Selvam et al., 2018; Shalini et al., 2015), and its suppression by BPE could prevent unnecessary fibroblast loss, preserving the cells required for collagen synthesis and extracellular matrix maintenance. As a member of the matrix metalloproteinase family, MMP-1 facilitates the breakdown of type I collagen, a primary structural protein in the skin (Potekaev et al., 2021). ROS-induced excessive expression of MMP-1 promotes ECM degradation, contributing to the accelerated aging of the skin, manifesting as increased wrinkling and a decline in skin elasticity (Freitas-Rodríguez et al., 2017; Liguori et al., 2018). The ability of BPE to suppress MMP-1 expression suggests its potential role in maintaining collagen integrity and slowing the photoaging process. The upregulation of COL1A1, a gene encoding type I collagen, indicates that BPE supports fibroblast activation and collagen production (Potekaev et al., 2021). A scientific investigation revealed that topical application of 15% BPE cream significantly inhibited the expression of MMP-1 in Wistar rats subjected to UVB radiation, thereby maintaining collagen levels in the skin (Parwata et al., 2023). Beetroot and aloe vera extracts can serve as key ingredients in an antiaging gel that effectively enhances skin moisture, minimizes pore size, and reduces wrinkles when used at an optimal concentration (Maimunah & Prayoga, 2023). This aligns with beetroot’s potential role in promoting skin regeneration and improving skin elasticity. Increased type I collagen production helps restore the extracellular matrix, counteracting the degenerative effects of UVB exposure and other stressors.
Figure 8 demonstrates the proposed molecular mechanisms underlying BPE shields against UV-driven oxidative damage and skin aging. Intrinsic and extrinsic contributors to skin aging, such as reduced antioxidant defense and UV exposure, accelerate the production of unstable oxygen species, causing an imbalance in cellular redox homeostasis (Hussen et al., 2025). This excessive oxidative burden impairs mitochondrial function and triggers key signaling routes such as NF-κB and MAPK (Anderton & Alqudah, 2022; Hussen et al., 2025; Liguori et al., 2018). NF-κB drives the generation of proinflammatory molecules, including interleukins (IL-1, IL-6) and tumor necrosis factor-alpha (TNF-α), while MAPK contributes to the upregulation of AP-1 and MMP-1, leading to collagen, elastin, and hyaluronic acid depletion, thereby accelerating extracellular matrix breakdown (Anderton & Alqudah, 2022; Ansary et al., 2021). Additionally, mitochondrial dysfunction triggers cytochrome c release, activating caspase-3, which promotes apoptosis and accelerates cellular senescence. Together, these mechanisms result in hallmark aging characteristics such as wrinkles, subtle folds, fine creases, and a decline in skin tone (Jin et al., 2023).
Figure 8
Proposed mechanism of BPE as a potential antiaging agent through its activities as an antioxidant, anti-inflammatory, and antiapoptotic agents.

Beetroot, rich in antioxidants such as betalains, flavonoids, and phenolics, intervenes by neutralizing ROS and restoring redox balance. This reduces oxidative stress, thereby suppressing the initiation and amplification of NF-κB and MAPK signaling mechanisms (Hussen et al., 2025). As a result, the post-signaling expression of inflammatory regulators (e.g. IL-6) and collagenase enzymes like MMP-1 is downregulated, preventing collagen degradation. Furthermore, BPE enhances mitochondrial function, which reduces cytochrome c emission and subsequently lowers caspase-3 activity, protecting cells from apoptosis. The extract also promotes COL1A1 expression, facilitating collagen synthesis and extracellular matrix repair. Beetroot also contains vitamin C, which could protect against oxidative damage by scavenging ROS and promote collagen synthesis by activating procollagen enzymes (Baião et al., 2020; Chen et al., 2021). Existing research also stated that the nitrate component in beetroot, when converted to nitric oxide, might improve microcirculation by enhancing blood flow and oxygen delivery to skin cells, thus improving nutrient delivery, oxidative stress, inflammation, and mitochondrial function (Baião et al., 2020; Chen et al., 2021; Clifford et al., 2015). Collectively, these mechanisms highlight beetroot’s ability to protect skin cells from oxidative damage while restoring cellular function and structural integrity, positioning it as a promising antiaging agent.
These findings reinforce BPE’s potential efficacy as an antiaging compound, particularly through its antioxidant activity and protective effects against skin aging. However, these results need further confirmation through future studies to identify the specific compounds present in BPE and to unravel the biological pathways associated with each active component, such as betalains, phenolics, and flavonoids, in protecting the skin from aging. Additional research is also required to measure both the bioactivity and safety implications of BPE in more complex skin models, such as in vivo studies in rats or clinical trials in humans.
5. CONCLUSION
This study highlights the potential role of BPE as an antiaging agent through its antioxidant, anti-inflammatory, and antiapoptotic properties. BPE effectively scavenges free radicals, as evidenced by its strong DPPH activity, and enhances TAOC in UVB-induced human fibroblast cells. BPE also inhibits elastase and HAase, key enzymes responsible for ECM degradation, thereby preserving skin elasticity and hydration. At the molecular level, BPE treatment led to a notable increase in COL1A1 gene expression, indicating its role in supporting collagen synthesis and skin regeneration. Simultaneously, it downregulated IL-6, Casp-3, and MMP-1 gene expression, reducing inflammation, apoptosis, and collagen breakdown—three critical factors in the skin aging processes.
These findings suggest that BPE acts as a natural modulator of oxidative stress and ECM integrity, making it a promising candidate for natural antiaging formulations. However, further studies are needed to identify the specific bioactive compounds responsible for these effects, optimize formulations for topical applications, and evaluate BPE’s long-term safety and efficacy in in vivo models and clinical settings.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest related to the publication of this review paper.
AUTHORS’ CONTRIBUTIONS
J.W.G.: Research concept and design, collection and/or assembly of data, data analysis and interpretation, and writing, critical revision, and final approval of the article; D.K.J.: Research concept and design, collection and/or assembly of data, writing, critical revision, and final approval of the article; S.T.: Collection and/or assembly of data, writing, critical revision, and final approval of the article; A.H.N.S.: Data analysis and interpretation, writing and final approval of the article; F.H.Z.: Data analysis and interpretation, critical revision, and final approval of the article; W.W.: Writing, critical revision, and final approval of the article.