1. INTRODUCTION
The uncontrolled growth of cells that have outgrown the body’s natural control systems is known as cancer (Krieghoff-Henning et al., 2017). Cancer is unique among diseases as it can be metabolic, inflammatory, genetic, or all of these at once (Gyamfi et al., 2022). Any tissue in the body can acquire cancer, but each one has its characteristics even though the fundamental mechanism is the same. In India, oral cancer poses a serious public health concern because of its unchecked cellular growth and invasion of nearby tissues in the oropharyngeal cavity. In India, oral squamous cell carcinoma (OSCC) is the most prevalent cancer that poses a threat to public health (Pires et al., 2013). OSCC typically presents as a tongue ulcer that does not heal, accompanied by varied degrees of xerostomia and mucositis. In addition, there could be fistula, limited mouth opening, ulcers, and lymphadenopathy (Minhas et al., 2017).
It is the sixth most common type of cancer worldwide. With an estimated one-third of all cases globally, India is disproportionately affected. A quarter of the world’s mouth cancer cases occur in India, where there are an astounding 77,000 new cases diagnosed each year and 52,000 related fatalities. About 70% of cases in India are detected at an advanced stage, in contrast to Western countries where early diagnosis is more prevalent. This considerably lowers the 5-year survival rate, which in India is still alarmingly low at about 20%. India has a higher risk of oral cancer because of several reasons. Smoking and nonsmoking tobacco use, excessive alcohol intake, poor dental hygiene, malnutrition, and persistent viral infections are recognized risk factors. Also, chewing paan is a significant local risk factor (Borse et al., 2020). In addition, the risk of oral cancer in this population is further increased by periodontal disease, which is frequently linked to the use of paan (betel leaf). The most commonly used treatment modalities are typically chemotherapy and radiation therapy, either before or following surgical excision of the malignant tumor. These therapeutic approaches frequently result in side effects such as mucositis, infection, taste malfunction of the salivary glands, and pain, which lead to secondary problems like malnourishment, dehydration, and dysgeusia (Minhas et al., 2017).
Head and neck radiation can cause irreversible damage to the oral mucosa and its blood vessels, which can lead to trismus, xerostomia, and bone and soft tissue necrosis (Borse et al., 2020). Because of the metastases, it is frequently impossible to surgically remove malignant growths (Tohme et al., 2017). According to a study, drug-resistant opportunistic bacteria developed in the oral cavity of elderly patients in China following treatment for oral cancer. In addition, it has been proposed that the presence of bacteria in the mouth cavity raises the risk of illnesses such as bacteremia and pneumonia (Pan et al., 2014). Therefore, a medication that can inhibit tumor cell growth, reduce the severity of symptoms, and aid in pain management must be used in the entire care of oral cancer. Numerous formulations in the Siddha school of medicine, which is indigenous to Southern India, offer substantial anticancer properties.
One such formulation is vaan mezhugu (VM), a Siddha herbo-mineral drug indicated for conditions such as Kandamaalai (Cervical lymphadenitis), Kiranthi (Glandular swelling), Purai (Sinus), and various other ailments (Kuppusamy, 1993; Sambasivampillai, 1991). VM is clinically used in the management of oral cancer by Siddha physicians, but currently, there is no scientific evidence for it. In addition, VM contains the following ingredients: Thaalagam (Yellow arsenic trisulfide), Veeram (Hydrargyrum perchloride), Pooram (Hydrargyrum subchloride), Lingam (Red sulfide of mercury), and Rasam (Hydrargyrum). Numerous scientific studies have already indicated the enormous potential of these ingredients in curing cancer. Therefore, this study aimed to evaluate VM’s growth-inhibiting impact in the human oral cancer KB cell line.
2. MATERIALS AND METHODS
2.1 Selection of the experimental drug
Siddha Vaithiya Thirattu written by Kuppusamy (1993), which is part of Siddha literature, indicates that the trial drug, VM, is one of the herbo-mineral formulations for Kaandamalai (Cervical lymphadenitis), Kiranthi (Glandular swelling), Purai (Sinus), Powthiram (Fistula), Manjal Kaamaalai (Jaundice), Gunmam (Peptic ulcer), and Kaasam (Bronchitis). The drug was procured from a GMP-certified pharma company.
2.2 Ingredients of VM
The components of VM are detailed in Table 1.
Table 1
Ingredients of vaan mezhugu
2.3 Preparation of VM
First, Gandhagam and Rasam were ground together into a fine black powder and separated. Subsequently, the other ingredients were added and mixed with the previously prepared Gandhagam and Rasam powder. Anda Thylam was then incorporated, and the mixture was ground thoroughly for 4 hours until it reached a semisolid, wax-like texture. The final product (VM) was stored in an airtight container.
Dosage: Ulunthalavu (65 mg)
Adjuvant: Palm jaggary
2.4 In vitro evaluation of anticancer activity
2.4.1Cytotoxic effect determination by MTT assay
The KB human oral cancer cell line, sourced from the “National Centre for Cell Sciences (NCCS), Pune, India, was cultured in 25 cm2 flasks using Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS, L-glutamine, sodium bicarbonate, and antibiotics (penicillin, streptomycin, and amphotericin B).” Cells were maintained at 37°C in a humidified incubator with 5% CO2. Viability was assessed visually using an inverted phase-contrast microscope and through the MTT assay.
After trypsinization, 5×103 cells/well were seeded in 96-well plates and incubated under standard conditions. The test compound was prepared by dissolving 1 mg in 1 mL of 0.1% DMSO, mixed with a cyclomixer, and filtered through a 0.22 µm filter. Cells were treated with the compound at concentrations of 100, 50, 25, 12.5, and 6.25 µg/mL in triplicates, while untreated cells served as controls.
Cytotoxic effects, including cell rounding, shrinking, granulation, and vacuolization, were observed microscopically. For the MTT assay, 30 µl of MTT solution was added after 24 hours of incubation. Following a 4-hour incubation, the supernatant was removed, formazan crystals were dissolved in 100 µl DMSO, and absorbance was measured at 540 nm using a microplate reader (Jerard et al., 2020; Mosmann et al., 1983).
2.4.2Assessment of apoptosis using acridine orange (AO) and ethidium bromide (EtBr) dual staining
KB human oral cancer cells were cultured following standard protocols and treated with the lethal concentration (LC50) of sample (21.69 µg/mL) for 24 hours, alongside untreated controls. After incubation at 37°C in a 5% CO2 humidified environment, the cells were washed with cold PBS and stained with a mixture of AO (100 µg/mL) and EtBr (100 µg/mL) for 10 minutes at room temperature. The stained cells were rinsed twice with PBS and examined under a fluorescence microscope “(Olympus CKX41 with Optika Pro5 camera)” using a blue filter. Cells were classified into four categories: live, early apoptotic, late apoptotic, and necrotic (Zhang et al., 1998).
2.4.3Apoptosis by annexin V flow cytometry
KB human oral cancer cells were cultured as per standard procedures and treated with the LC50 of the sample (21.69 µg/mL) from a 1 mg/mL stock solution, with untreated controls maintained. Cells were incubated at 37°C in a humidified 5% CO2 incubator for 24 hours. Following incubation, the cell sample was transferred to a 12×75-mm polystyrene tube, ensuring a minimum of 1×106 cells for fixation. Samples were centrifuged at 3000 rpm for 5 minutes, and the supernatant was carefully removed without disturbing the pellet.
Next, 100 µL of Muse™ Annexin V and a dead cell reagent was added to each tube. The mixture was gently vortexed or pipetted for 3–5 seconds and incubated for 20 minutes at room temperature in the dark. Finally, the cells were analyzed using a flow cytometer with Muse FCS 3.0 software, comparing them to untreated controls to assess apoptosis (Ciniglia et al., 2010).
3. RESULTS
3.1 Evaluation of in vitro cytotoxicity using the MTT assay
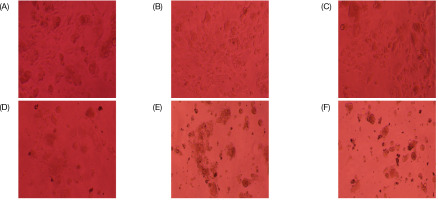
Cytotoxic effects of VM on cancer cells at different concentrations show a dose-dependent decrease in cell viability (Figure 1).
Figure 1
Cancer cells exposed to varying concentrations of VM. (A) Control, (B) VM–6.25 µg/mL, (C) VM–12.5 µg/mL, (D) VM–25 µg/mL, (E) VM–50 µg/mL, and (F) VM–100 µg/mL.
Cytotoxic effects of VM on cancer cells treated with various concentrations of cell viability percentage are presented in Table 2 and Figure 2.
Table 2
Percentage viability of vaan mezhug at various concentrations.
| VM Concentration µg/mL | Percentage Viability |
|---|---|
| Control | 100 |
| 6.25 | 91.84*** |
| 12.5 | 71.72*** |
| 25 | 42.19*** |
| 50 | 30.97*** |
| 100 | 17.12*** |
| ***p < 0.001 | |
Figure 2
A graph illustrating the anticancer effect of the sample on KB cells using the MTT assay was plotted.
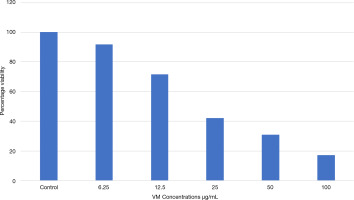
The Y-axis represents percentage cell viability, while the X-axis shows different sample concentrations. All experiments were conducted in triplicates, and results are presented as Mean ± SE. Data were analyzed using one-way ANOVA followed by Dunnett’s test, with ***p < 0.001 considered significant compared to the control group.
3.2 Determination of apoptosis by AO and Etbr double staining
The cells were categorized into four groups:
3.3 Apoptosis by annexin—V flowcytometry
VM treatment induces apoptosis in cancer cells, as evident from the significant differences observed between the untreated groups, as shown in Figure 4 and Table 3.
Table 3
Cell distribution of cells in control cells.
| Live (LL) | 2.44E+05 | 88.69 |
|---|---|---|
| Early Apoptotic (LR) | 1.17E+04 | 4.26 |
| Late Apoptotic/Dead (UR) | 1.52E+04 | 5.54 |
| Debris (UL) | 4.16E+03 | 1.51 |
| Total Apoptotic | 2.69E+04 | 9.80 |
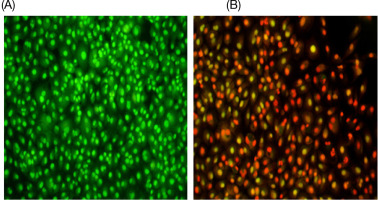
The percentage of early apoptotic cells was 4.26%, while the late apoptotic cells accounted for 5.54%. The overall apoptotic percentage was low, totaling 9.80%.
VM treatment induces apoptosis in cancer cells, as evident from the significant differences observed between treated groups, as shown in Figure 5 and Table 4.
Table 4
Cell distribution in cells treated with vaan mezhug.
| Parameter_Cell Concentration (Cells/mL)_% Gated_ | ||
|---|---|---|
| Live (LL) | 3.68E+05 | 66.98 |
| Early Apoptotic (LR) | 1.39E+04 | 2.52 |
| Late Apoptotic/Dead (UR) | 1.14E+05 | 20.78 |
| Debris (UL) | 5.35E+04 | 9.72 |
| Total Apoptotic | 1.28E+05 | 23.30 |
In the VM-treated cells, the early apoptotic percentage was 2.52%, while the late apoptotic percentage was 20.78%. In addition, 9.72% of the cells were debris, leading to a total apoptosis rate of 23.30%.
4. DISCUSSION
Chemotherapy and radiotherapy play vital roles in the management of oral cancer. Such treatment results in adverse physical, psychological, and economic effects (Bjordal et al., 1994). Common physical effects such as nausea, vomiting, diarrhea, fatigue, and generalized weakness are persistent and affect daily activities (Karthikeyan et al., 2012). Cognitive impairments such as concentration and memory loss are not uncommon. Functionally, post-treatment causes changes in swallowing and chewing (Gunn et al., 2013). Consequences of surgery and radiotherapy such as facial impairment, disfigurement, and less lip mobility drastically lower the self-esteem of the patient (Bhandari et al., 2024; Mishra et al., 2024).
A decline in “health-related quality of life (HRQL)” is seen in long-term cancer patients (Nordgren et al., 2003). Psychologically, extreme levels of depression and anxiety are observed, but fear of recurrence is consistent and, in turn, affects mental health (Ryan et al., 2005; Reyes et al., 2023). Economically, patients are affected because of treatment costs which results in financial strain and thoughts of committing suicide (Wajid et al., 2021). It is therefore essential to explore alternative therapies that are effective while minimizing side effects. Presently, cancer research emphasizes both modern medical treatments and traditional healing practices. A holistic approach that integrates conventional therapies with traditional remedies could prove vital in tackling this complex disease.
The Siddha system of medicine is grounded in three core principles: vali (movement), azhal (transformation), and iyam (structure). Specifically, azhal plays a crucial role in cellular digestion and metabolism. An imbalance where azhal dominates over iyam (fire surpassing water) is a hallmark of tumors, resulting in unchecked tissue growth. Conversely, a deficiency in azhal can cause an imbalance favoring vali and iyam, potentially leading to issues such as tissue migration because of disruptions in Viyana vaayu, Samana vaayu, and Suzhumunai naadi.
Each person has a unique constitution, referred to as Thegi, which is influenced by the dominant dosham “(Vaatham, Pitham, Kapham, or combinations thereof)” and the individual’s body time. This constitution determines how a person interacts with their environment, their susceptibility to diseases, and their response to treatments. To maintain health, it is essential to keep the dosham in balance. In the context of cancer, the most affected doshas, Kapham and Vaatham, often exacerbate tumor growth and spread (Subathra et al., 2022). Various compound formulations have been suggested for the treatment of oral cancer, including VM, a preparation made from multiple ingredients. This formulation works by addressing imbalanced vital forces, restoring harmony through its inherent heat-based potency.
The results of the MTT assay demonstrated that cell viability decreases as drug concentration increases, exhibiting an inverse relationship. At a concentration of 6.25 µg/mL, cell viability was 91.84%, whereas at 100 µg/mL, it dropped significantly to 17.12%. These findings confirm that the cytotoxic effect of the drug is dose-dependent. LC50, where 80% of cells were inhibited, was determined to be 21.6945 µg/mL.
Using the EtBr and AO double-staining method, the nuclei in the control group appeared green under epifluorescence, showing a uniform spherical structure and organized chromatin. In contrast, cells treated with the LC50 displayed distinct nuclear apoptotic bodies with fragmented DNA, visible as green fluorescent patches indicative of early apoptosis. In advanced stages of apoptosis, these apoptotic bodies disintegrated, resulting in fragmented DNA typical of late apoptosis, observed as orange to red-stained nuclei. Necrotic cells were identified by uniformly orange-stained nuclei.
Annexin V binding assays, in combination with propidium iodide, were employed to differentiate apoptotic cells from necrotic cells, providing a reliable method for assessing apoptosis in testicular cancer cells. Quantification revealed that the fold increase in cell death compared to the control reflected the apoptotic response to treatment with the LC50. Flow cytometry analysis using annexin V detailed the cellular response to therapy, highlighting the effectiveness of the treatment. Results showed that late apoptotic changes increased from 5.54% in the control group to 20.78% in the drug-treated group. The total percentage of apoptotic cells rose from 9.80% in the control to 23.30% in the treated group, underscoring the drug’s significant role in inducing apoptosis and enhancing cell death.
Certain components in this formulation, including Rasam (Hydrargyrum), Veeram (Hydrargyrum perchloride), Pooram (Hydrargyrum subchloride), Thaalagam (Yellow arsenic trisulfide), Lingam (Red sulfide of mercury), and Gandhagam (Sulfur), have been shown to exhibit cytotoxic and anti-proliferative properties. Manjari et al. (2018) studied the anticancer effects of Pancha paasana chendhuram (PPC), which contains Rasa chendoorum, Veeram, Vellai pasanam, Thalagam, Gaandham, and Gandhagam on MCF-7 cells, demonstrating dose-dependent cytotoxicity (IC50 = 59.26 µg/mL), apoptosis induction, Bax and p53 upregulation, Bcl2 downregulation, and moderate antioxidant activity, suggesting apoptosis induction via mitochondrial pathways (Manjari et al., 2018). Similarly, Abinaya (2018) evaluated the in vitro anticancer activity of Kalamega narayana chendoorum (KNC), containing Rasam, Lingam, Thalagam, and Gandhagam, against human KB oral cancer cells using cell viability and apoptosis assays (Abinaya, 2024). Manjari et al. (2016) investigated Rasa karpoora kuligai (RSK), with Pooram as the primary ingredient, on HeLa cells, showing reduced cell growth and dose-dependent regulation of apoptotic pathways (Manjari et al., 2016), while Rajalakshmi et al. (2014) demonstrated that VM, containing Veeram, exhibited 74.05% cytotoxicity against EAC cell lines at a concentration of 1000 µg/mL (Rajalakshmi, et al., 2013). This study used MTT assay, apoptosis double staining, and annexin V flow cytometry to show that VM plays a major role in its anticancer effects.
5. CONCLUSIONS
The test drug VM significantly reduced cell viability in a dose-dependent manner, indicating its cytotoxic effects. Images showed chromatin condensation and orange staining, confirming that apoptosis had occurred. Flow cytometry results revealed a notable percentage of late apoptosis in the treated group, with the LC50 value highlighting its ability to induce apoptosis to some extent. Furthermore, in vivo studies and clinical trials are needed to confirm its potential as a treatment for oral cancer.
ACKNOWLEDGEMENT
The authors are grateful for the support and facilities provided by the National Institute of Siddha, Tambaram sanatorium, and the Center for Research on Molecular Biology and Applied Science, Trivandrum, for their support in this study.