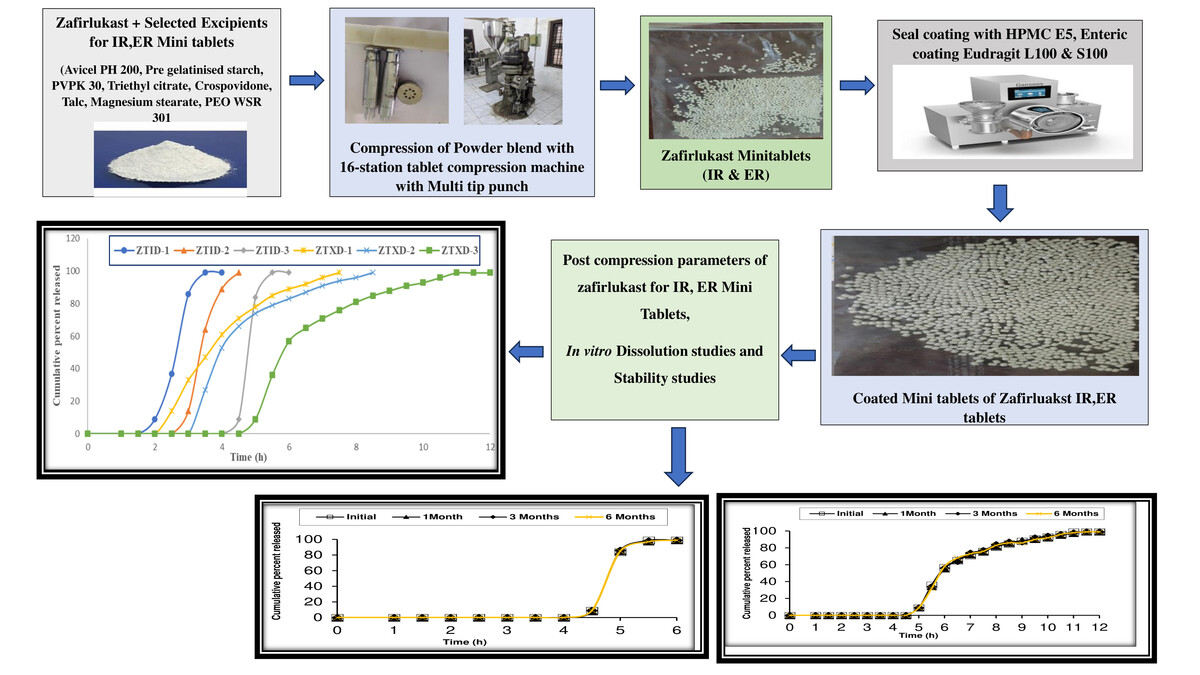
1. INTRODUCTION
A pulsatile release profile is one in which the drug is released quickly after a programmed lag time, which is useful when applying multiple therapies to treat therapeutic needs involving the circadian rhythm of disease or body functions (Jaiswal et al., 2019). Asthma is a chronic inflammatory disease of the airways that is characterized by hyperresponsiveness to a range of stimuli. Because circadian rhythms are important in the etiology and for the treatment of asthma, chronotherapy attempts to optimize the advantages of bronchodilator medications in the early morning hours. The pulsatile systems were created as a time-release pattern for the medication, which are helpful in reducing the frequency of doses, improving patient compliance, and decreasing asthma attacks in the early morning hours (Amit et al., 2012; Anusha et al., 2023).
Zafirlukast, a selective and competitive leukotriene (LT) D4 and E4 (LTD4 and LTE4) receptor antagonist, is one of the constituents of slow-acting substances that can induce anaphylaxis. Changes in cellular activity, smooth muscle contraction, airway edema, and the inflammatory process have all been linked to cysteine LT and receptor occupancy in the pathophysiology of asthma. (Neeharika et al., 2021). The coated mini tablets can precisely regulate when and where a medicine is delivered in the body—they are important for the pulsatile drug administration. This is especially helpful for diseases that have a circadian rhythm. Here, we formulated mini tablets of Zafirlukast coated for immediate release (IR) and extended release (ER) with Eudragit L100 and Eudragit S100 polymer to achieve the desired lag time.
2. MATERIALS AND METHODS
2.1. Materials
Zafirlukast has been supplied by the Aurobindo Pharma Limited and Avicel PH (pharmaceutical grade) 200, pregelatinized starch, polyvinylpyrrolidone (PVPK) 30, triethyl citrate, crospovidone, Eudragit L100, Eudragit S100, polyethylene oxide water-soluble resin (PEO WSR) 301 and hydroxypropyl methylcellulose (HPMC) E5 were obtained from Therallen Pharma Pvt Ltd, Hyderabad, all as gift samples. Talc, Aerosil and magnesium stearate were obtained from HiMedia Laboratories and Merck Chemicals Limited, Mumbai, India, respectively. All the remaining chemicals used were of analytical grade.
2.2. Methods
2.2.1. Preparation of immediate release mini tablets
The mini tablets of Zafirlukast IR (ZTI-1, ZTI-2, and ZTI-3) were prepared using compressible excipients such as Avicel PH 200, crospovidone, pregelatinized starch, and PVPK 30. Then, Zafirlukast and the defined excipients were uniformly mixed and passed through the American Society for Testing Materials (ASTM) mesh no 40 (425 μm) sieve. Aerosil 200, talc and magnesium stearate were utilized to lubricate the dry mix blend. Using a 3 mm multi-tip, round shaped punch, formulations were compressed on the 16-station tablet compression machine (Cadmach Machinery Co., Vatva, Ahmedabad, India). The formulations are summarized in Table 1.
Table 1
Formulations of Zafirlukast IR and ER mini tablets.
2.2.2. Preparation of extended release mini tablets
The mini tablets of Zafirlukast ER (ZTX-1, ZTX-2, and ZTX-3) were prepared using directly compressible excipients such as Avicel PH 200 and PEO WSR 301. They were mixed thoroughly, passed through the ASTM mesh no 40 (425 μm) sieve, and blended until uniformity was achieved. Aerosil 200, talc and magnesium stearate were utilized to lubricate the dry mix blend. Using 3 mm multi-tip, round shaped punch, formulations were compressed on the 16-station tablet compression machine (Cadmach Machinery Co.). The formulations are summarized in Table 1.
2.2.3. Coatings on prepared Zafirlukast IR and ER mini tablets
Seal Coating: The selected IR and ER mini tablets (ZTI-3 and ZTX-3) were first given 7% of HPMC E5 as seal coating. HPMC E5 and talc were mixed well in purified water to ensure uniform dispersion of the liquid. The prepared mini tablets were loaded on to the 12-inch Gansons coating pan. The inlet temperature was fixed at 60°C ± 2°C and the bed temperature was monitored at 45°C ± 1°C. The spray revolutions per minute (RPM) of the peristaltic pump was adjusted and monitored to avoid twinning. The formulations are summarized in Table 2.
Table 2
Seal coating on selected Zafirlukast IR and ER mini tablets.
Enteric Coating: The prepared seal coating in the mini tablets of IR and ER were further coated with enteric polymers a combination of 30%, 40%, and 50% of Eudragit L100 and Eudragit S100; ZTID-1, ZTID-2, and ZTID-3 and ZTXD-1, ZTXD-2, and ZTXD-3 with triethyl citrate and talc dispersed in either water or ethanol as solvents. Then they were loaded on to the 12-inch Gansons coating pan. The inlet temperature was fixed at 60°C ± 2°C and the bed temperature was monitored at 45°C ± 1°C. The spray RPM of the peristaltic pump was adjusted and monitored to avoid twinning. The formulations are summarized in Table 3.
Table 3
Enteric coating on the seal-coated Zafirlukast selected IR and ER mini tablets.
2.2.4. Evaluation of formulations
UV analytical method: To obtain 100 µg/mL of stock solution, a precise amount of 10 mg of the drug was transferred in to an 100 mL volumetric flask with ethanol. To get different concentrations of 5 μg/mL, 10 μg/mL, 15 μg/mL, 20 μg/mL, 30 μg/mL, and 40 μg/mL with HCl buffer pH 1.2 and of 5 μg/mL, 10 μg/mL, 15 μg/mL, 20 μg/mL, and 25 μg/mL with phosphate buffer pH 6.8, aliquots of prepared solution were poured into a 10 mL volumetric flask using a pipette. At λmax (maximum absorbance), it was measured against the corresponding blank buffer solution. In the pH 6.8 phosphate buffer, λmax was found to be 238 nm, 232 nm which was observed in acidic media 0.1N HCl (Krishna et al., 2021).
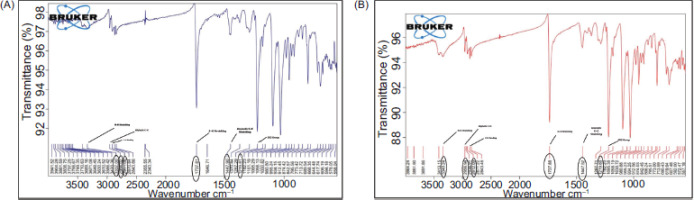
2.2.5. Drug excipient compatibility study
To find the drug with the selected excipients compatibility using a Bruker FTIR (Fourier Transform Infrared) Spectrophotometer, FTIR was done across a wavelength of between 4000–400 cm-1. The sample was incorporated with potassium bromide (KBr) and pressed into discs using a hydraulic press for 5 minutes under 5 tons of pressure; the pellet was analyzed to obtain the spectrum (Matkovic et al., 2005; Ranjan et al., 2014).
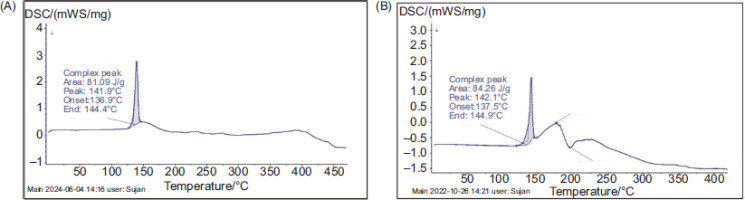
A DSC-60 (Shimadzu, Tokyo, Japan) was used to perform the differential scanning calorimeter (DSC). The samples were kept in a 40 µl aluminum pan 0.1 mm thick. The pan was then sealed by a perforated aluminum cover and heated to 250°C using nitrogen flow at a rate of 5°C/minute (Barde et al., 2023; Elmubarak et al., 2021).
2.2.6. Evaluation of preformulation parameters
Angle of repose (Daravath et al., 2014): Utilizing the funnel approach, one can compute the angle of repose of blended mix and ascertain that the greatest angle can be created among powder pile surface and horizontal plane. The formula to calculate the angle of repose is:
Where r and h are radius and height of the powder blend pile.
Bulk and tapped density (Momin et al., 2017): To observe the initial volume (fluff volume) from each formulation, 10 gm of powder blend was transferred in to a 50 mL measuring cylinder. At intervals of 2 seconds, Then the cylinder was permitted to descend onto the surface under its own weight from a height of 2.5 cm. The tappings were kept going and noted until there was no more change in volume. Finally, fluff and tapped density were determined by:
Where M = mass of the powder blend, Vi and Vt are powder blend initial volume and final volumes.
Hausner’s ratio and Carr’s Index (Momin et al., 2017): Carr’s Index, or Compressibility Index was calculated by using the following formula:
2.3. Evaluation of tablet properties
2.3.1. Content uniformity and weight variation
This test ensures the product’s quality, safety, and identity and also confirms that the dosage unit is uniform. The uniformity of weight of each tablet was determined in order to make sure that designed tablet was having the appropriate quantity of drug. Weighing every tablet, estimating their mean weight, and comparing those weights to the average mass constitute the United States Pharmacopeia (USP) weight variation test (Mhaske et al., 2024).
2.3.2. Tablet thickness
The tablet thickness is crucial for a uniform tablet size. Vernier calipers or a computerized caliper was measured using thickness (Aboutaleb et al., 2016).
2.3.3. Tablet hardness
Hardness is the amount of force applied on its diameter to break the tablet. It is helpful for determining the tablets resistance in transportation or breakage under storage conditions. Monsanto hardness tester was used to determine the hardness of the tablet in each batch and specified in terms of kilogram per square centimeter (kg/cm2). In testing for hardness, three formulations were chosen and recorded (Kumar et al., 2020).
2.3.4. Friability
After weighing the 20 tablets, we recorded the weight of tablet W1 and then put in a Roche Friabilator and processed at 25 RPM for 100 revolutions. The fines (small powder particles) and the tablets were gathered from the friabilator and again weighed and recorded as W2. The percentage friability was determined by using the equation (Syed et al., 2022):
2.3.5. Disintegration time
The test for the absorption of drug in plasma was conducted using the USP disintegration test instrument of Electrolab (Goregaon East, Mumbai, Maharashtra)—six glass tubes 3 inches long and open at the top and a 10-mesh screen at the bottom end. With every tube holding a tablet, the basket was placed in a 1 L beaker having 6.8 pH phosphate buffer 37 ± 1°C remaining 2.5 cm under the liquid’s surface and then the disintegration time was recorded (Kumar et al., 2020).
2.3.6. Content Uniformity
The randomly chosen tablets were weighed and powdered in a 100 mL volumetric flask. 20 mg dissolved in 5 mL of methanol/ethanol and sonicated for 5 minutes. Finally, the solution was mixed with phosphate buffer pH 6.8 and No. 41 Whattman’s filter paper was used to remove undissolved matter. after the serial dilutions were conducted, the absorbance at 238 nm were measured (Anusha et al., 2017).
2.3.7. In vitro dissolution studies
USP Type 1, known as the Basket method, a dissolution testing technique, was used (Labindia DS 8000, India) and the study was performed at 37°C ± 0.5°C at 50 RPM. In vitro dissolution studies for the tablets of IR were carried out with phosphate buffer pH 6.8 (using SLS [sodium lauryl sulfate] of 0.5%). For the enteric-coated tablets of IR, the first 2 hours of dissolution was performed in a 500 mL HCl of 0.1N (using SLS of 0.5%) and then by using phosphate buffer, pH 6.8 (using SLS of 0.5%). The aliquots were taken at specified time intervals and each 5 mL of sample was replaced by the freshly prepared medium. The samples prepared were determined by UV Visible spectrophotometer at λmax recorded at 238 nm in 6.8 phosphate buffer and 232 nm in acidic media 0.1N HCl (Krishna et al., 2021; Ravi et al., 2020).
2.3.8. Stability studies
For the optimized formulations of the enteric-coated IR, ER mini tablets were selected, filled, sealed in a 100 CC high-density polyethylene (HDPE) container. Three sets of each formulation were prepared and stored in a stability chamber for 6 months at 40°C ± 2°C/70% RH ± 5% RH. The samples were evaluated at 1, 3, and 6 months for drug content, color change, and drug release (Kulkarni et al., 2011; Ahmad et al., 2023).
3. RESULTS AND DISCUSSION
3.1 Drug-excipient compatibility
The formulations, analyzed with the help of FTIR and DSC studies, showed that there were no appreciable chemical interactions between the drug and incorporated excipients. These findings are illustrated in Figures 1 and 2.
3.2. Pre-compression parameters of Zafirlukast IR and ER Tablets
Pre-compression studies were conducted on all prepared formulations with satisfactory results. The bulk density and tapped density values were within acceptable limits, ranging from 0.348 ± 0.21 to 0.392 ± 0.09 g/cm3 and 0.390 ± 0.27 to 0.440 ± 0.11 g/cm3, respectively. Based on these values, the Hausner’s ratio and Compressibility Index were calculated and found to be within acceptable ranges: 1.12 ± 0.11 to 1.16 ± 0.32 for Hausner’s ratio and 10.82 ± 0.23 to 14.36 ± 0.11 for the Compressibility Index, indicating good flow properties of the powder blends. Additionally, the angle of repose, which ranged from 32.12 ± 0.24° to 34.23 ± 0.32°, further supports the observation of good flowability.
3.3. Post-compression parameters of Zafirlukast IR and ER mini tablets
3.3.1 Thickness
The IR and ER mini tablet thickness of Zafirlukast was determined to vary between 2.31 ± 0.11 mm and 2.89 ± 0.24 mm. The variation recorded is within permissible pharmaceutical limits, demonstrating tablet compression uniformity between the formulations. Uniform thickness is required to support proper drug content accuracy and equal coating, especially in multiparticulate systems such as the mini tablets.
3.3.2 Weight variation test
The tested Zafirlukast IR and ER mini tablets complied with the pharmacopoeial standards, as the percentage weight variation remained within acceptable limits. This confirms uniformity in tablet weight and consistency in the manufacturing process.
3.3.3. Hardness test
For IR mini tablets, the results showed a hardness of 2.1 ± 0.21 – 2.4 ± 0.11 kg/cm2, while for ER mini tablets, the range was 2.4 ± 0.09 – 2.9 ± 0.13 kg/cm2. Every formulation showed sufficient mechanical strength to endure handling, coating, and packaging procedures without breaking.
3.3.4. Disintegration test
For the disintegration test of the core tablets, the result was 2 ± 0.11 – 4 ± 0.23 minutes in IR Tablets. These results fall well within the acceptable limits specified for immediate release tablets, indicating that the formulations are capable of breaking down rapidly after administration. The observed disintegration times reflect good formulation design and the effective selection of disintegrants.
3.3.5. Friability test
The percentage of friability ranged between 0.48 and 0.66, indicating that the tablets were mechanically stable. A percentage of friability below 1 is mostly acceptable since it shows that the tablets are not easily broken, chipped, or abraded when handling, packaging, and transporting.
3.3.6. Content uniformity
The percentage of drug content for the Zafirlukast formulation was found to range from 98.24 ± 0.52 to 100.12 ± 0.08 in the IR and ER tablets and from 99.12 ± 0.35 to 99.63 ± 0.24 in the enteric-coated tablets. These are within the acceptable range according to the pharmacopoeial standards, indicating uniform distribution of the drug in all the tablets. The uniformity in drug content also validates the process of manufacture as reliable, in that each tablet will impart the desired dose with little to no variation.
The obtained results of the post-compression parameter tests are illustrated in Table 4.
Table 4
Post–compression parameters of Zafirlukast IR and ER mini tablets.
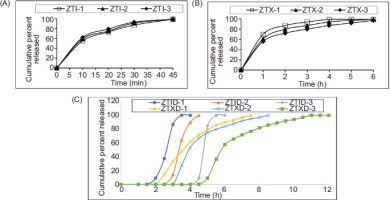
3.3.7. In vitro dissolution studies
The in vitro dissolution studies of ZTI-1, ZTI-2, and ZTI-3 indicated 99% drug release in 45 minutes, although the results revealed ZTI-3 of rapid drug release. Under Extended drug release ZTX-3 revealed more effectiveness compared to ZTX-1, ZTX-2. They also indicated release of the drug immediately after the lag time; hence, ZTID-3 indicated a 4-hour lag time with 99% drug release at the 6th hour. The studies also revealed the ER profile of the drug after lag time; hence, ZTXD-3 indicated a 4.5-hour lag time with 99% drug release at the 11th hour. The outcomes are depicted in Figure 3.
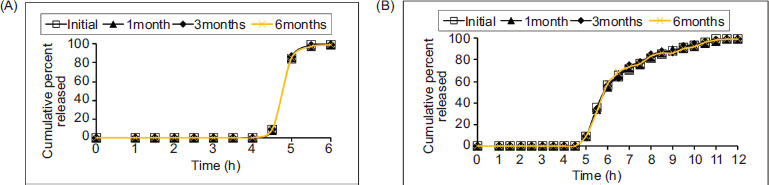
3.3.8. Stability studies
Stability studies were performed on optimized formulations ZTID-3 and ZTXD-3 in a stability chamber for 6 months at 40°C ± 2°C/70% RH ± 5% RH. Aliquots were taken out at 1, 3, and 6 months. The optimized formulation was stable as indicated by nonoccurrence of deviation in the percentage of the drug content, change in color, and in the percentage of drug release. The results are shown in Figure 4.
3.3.3.1. Photographic images of IR and ER mini tablets
The prepared formulations of the mini tablets photomicrographic image are illustrated in Figure 5.
4. CONCLUSION
In this study, an efficient pulsatile drug delivery system was successfully developed using Zafirlukast as the active pharmaceutical ingredient. The formulations were designed as mini tablets with both IR and ER profiles. The IR tablets were designed to release the drug immediately after a specified lag time, while the ER tablets were formulated to release the drug gradually after an initial lag period. The incorporation of enteric coatings using Eudragit L100 and Eudragit S100 in the Zafirlukast mini tablets effectively delayed the drug release, with the drug being released only after the lag time. Among the optimized formulations, ZTID-3 exhibited a 4-hour lag time with 99% drug release at the 6th hour, while ZTXD-3 showed a 4.5-hour lag time with 99% drug release at the 11th hour. The characterization and compatibility study of the designed system reveals the stability of the drug in prepared formulations. Thus, based on the above results, it can be stated that the polymers can be used to formulate a pulsatile drug delivery system for enteric-coated mini tablets of Zafirlukast.
ACKNOWLEDGMENTS
The authors gratefully acknowledge GITAM School of Pharmacy and A.K.R.G College of Pharmacy, Nallajerla, for their continuous support and cooperation throughout the research.
AUTHOR CONTRIBUTIONS
Research concept, Study design, methodology, collecting data, analyzing the results, investigation, and writing: P.S.; Conceptualization, supervision: L.S. All authors reviewed results and approved the final version of the manuscript. Both authors have contributed equally toward the present research work and manuscript.