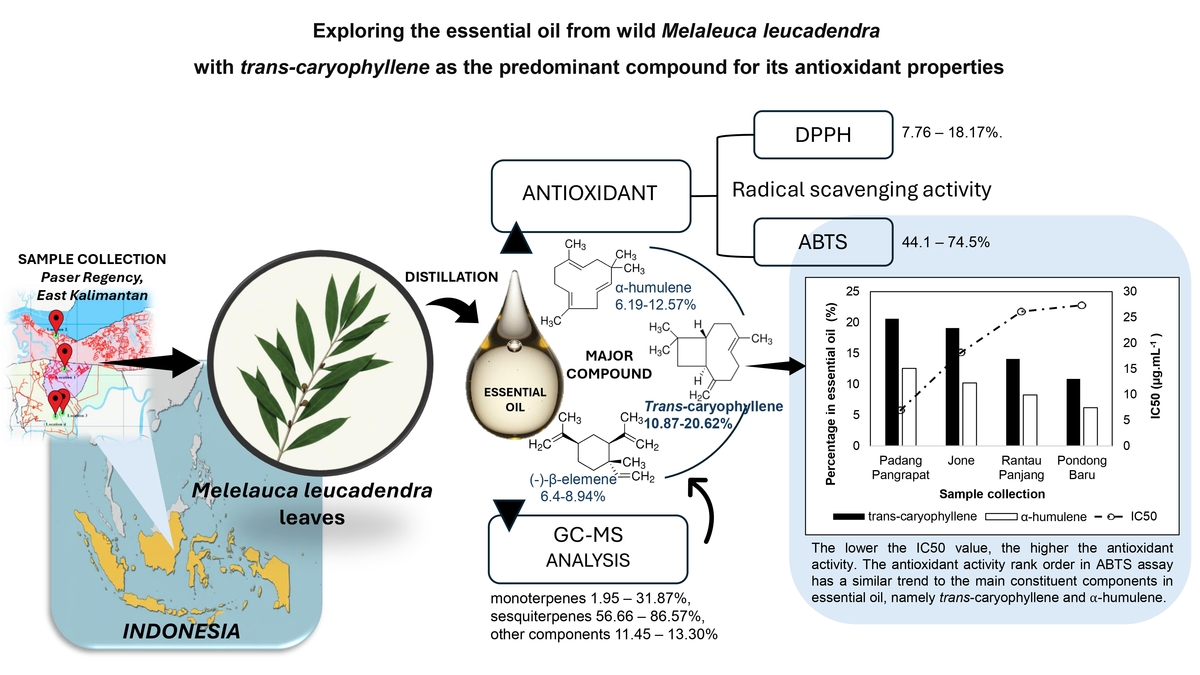
1. INTRODUCTION
Melaleuca, a genus within the Myrtaceae family, is a well-known aromatic and medicinal plant prized for its essential oils. These species naturally occur in open forests, woodlands, and shrublands, often near watercourses and swamp edges (Barbosa et al., 2013; Sciarrone et al., 2010). Melaleuca species are widely recognized for their distinctive aromatic properties and their significant economic importance, particularly in the essential oil industry (Padalia et al., 2015). Furthermore, these species display extensive phenotypic diversity and have demonstrated a capacity to adapt to climate change through Wright’s “Migration Adaptation” model, allowing them to thrive in a range of ecological conditions (Tran et al., 2013).
Across different geographical regions, Melaleuca species exhibit considerable variability in both species diversity and essential oil composition. Studies from India, New Caledonia, Tunisia, South Africa, China, Indonesia, and Brazil indicate that Melaleuca essential oils predominantly consist of terpene compounds, particularly monoterpenes, sesquiterpenes, and their associated alcohols (Amri et al., 2012; Hnawia et al., 2012; Joshi et al., 2021; Liao et al., 2017; Oyedeji et al., 2014; Sutrisno et al., 2018). Among the key constituents commonly found in Melaleuca oils are methyl eugenol, 1,8-cineole, terpinen-4-ol, viridiflorol, α-terpinene, caryophyllene, and caryophyllene oxide, all of which contribute to their biological activities.
Notably, trans-caryophyllene (TC) has emerged as a major bioactive sesquiterpene with pharmaceutical potential, known for its anti-inflammatory, analgesic, antioxidant, and antimicrobial properties (Gertsch et al., 2008; Jang et al., 2020; Wang et al., 2020). As a CB2 receptor agonist, trans--caryophyllene is particularly effective in reducing inflammation and alleviating pain without psychoactive effects, making it a promising therapeutic candidate for arthritis and neuropathic pain (Fidyt et al., 2016; Legault and Pichette, 2010). TC has garnered increasing attention in recent clinical and preclinical studies due to its diverse pharmacological properties. As a selective CB2 receptor agonist, TC exhibits significant anti--inflammatory effects without psychoactive consequences, positioning it as a potential therapeutic agent for inflammatory disorders (Rahimi et al., 2023). Moreover, trans-caryophyllene exhibits anticancer and neuroprotective effects, making it relevant in Alzheimer’s and Parkinson’s disease treatment (Zhang et al., 2017; Ramos-Molina et al., 2023). Additionally, TC has been shown to enhance glucose uptake and metabolic regulation, making it a promising candidate for managing metabolic disorders, including diabetes and obesity (Geddo et al., 2021). Beyond its internal therapeutic benefits, TC has broad-spectrum antimicrobial properties, contributing to its potential as a natural preservative in food and pharmaceutical applications (Angane et al., 2022). In addition to its health benefits, trans--caryophyllene also possesses broad-spectrum antimicrobial activity, further enhancing its potential applications in wound healing, infection control, and food preservation (Kasujja, 2021).
In Indonesia, Melaleuca is commonly referred to as “Kayu Putih,” and its essential oil—commonly known as cajuput oil—is primarily derived from M. cajuputi and M. leucadendra (or M. leucadendron). However, early references to M. leucadendra or M. leucadendron in cajuput oil production often actually referred to M. cajuputi (Giesen and Sari, 2018; Widiana, et al., 2015). While M. cajuputi is widely cultivated for commercial oil production, M. leucadendra is typically found in South and Central Kalimantan, Sumatra, Java, Maluku, Papua, and peatland regions. The ability of M. leucadendra to thrive in peatland environments, which have distinct ecological and soil characteristics compared to mineral soils, further highlights its adaptability and potential for sustainable cultivation (Idrus et al., 2020; Pujiarti et al., 2011; Torry and Idrus, 2016).
The biological activity of Melaleuca essential oils has been extensively documented, with studies confirming their antibacterial, antiprotozoal, anti-inflammatory, antifungal, insecticidal, anti-termite, and antiviral properties (Ardiana, 2021; Bakar et al., 2019; Chidi et al., 2020; Kasujja, 2021; Roszaini, et al., 2013; Usachev et al., 2013). These essential oils have also been recognized as natural antioxidants, contributing to their potential use in pharmaceuticals, cosmetics, and food preservation (Siddique et al., 2020; Wang et al., 2020). As the demand for natural antioxidants continues to rise, the exploration of Melaleuca essential oils presents significant commercial and therapeutic potential.
Despite extensive research on 1,8-cineole-rich chemotypes, limited studies have focused on the chemical diversity of trans-caryophyllene-rich M. leucadendra essential oils. This study aims to address this knowledge gap by conducting a comprehensive analysis of the essential oil composition of wild M. leucadendra, with a particular focus on trans-caryophyllene as a primary constituent. Understanding the environmental and biological factors influencing trans-caryophyllene concentration is crucial for enhancing its pharmaceutical applications, optimizing sustainable cultivation practices, and expanding its commercial utilization. Furthermore, considering that only a few studies on essential oils from East Kalimantan have been conducted (Kartiko et al., 2021; Kuspradini et al., 2019, 2022, 2018, 2020, 2023, 2016, 2021; Putri et al., 2018; Silau et al., 2021), this study seeks to evaluate the chemical diversity and bioactivity of M. leucadendra essential oil from this region. Given the variability in essential oil composition due to botanical and environmental factors, this study also aims to assess the antioxidant activity of M. leucadendra essential oil.
In this study, the antioxidant capacity of essential oil samples was assessed using only the DPPH (2,2--diphenyl-1-picrylhydrazyl) and ABTS (2,2'--azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) assays due to their complementary mechanisms and practical advantages for screening complex, lipophilic mixtures. DPPH effectively measures hydrogen atom transfer (HAT) from antioxidants such as phenolic compounds prevalent in essential oils, while ABTS captures both HAT and single electron transfer (SET), providing a broader evaluation of radical scavenging capacity (Prior et. al, 2005). These assays are simple, cost-effective, and adaptable to organic solvents, making them suitable for essential oils compared to resource-intensive methods like ORAC or the Lipid Peroxidation Assay, which require specialized equipment or lipid substrates (Apak et al., 2016). The FRAP assay was excluded as it focuses solely on reducing power in an aqueous system less compatible with hydrophobic essential oils (Moon & Shibamoto, 2009).
By comparing TC-rich essential oils derived from M. leucadendra samples collected across multiple locations in East Kalimantan and identifying the primary antioxidant contributors, these findings will contribute to optimizing M. leucadendra essential oil applications in medicine, pharmaceuticals, and food preservation, supporting its potential commercialization as a natural antioxidant and therapeutic agent.
2. MATERIALS AND METHODS
2.1. Materials and methods
2.1.1. Materials
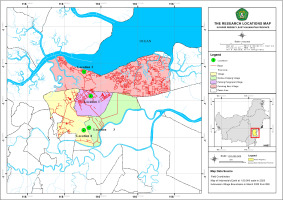
Study area. The plant samples used were M. leucadendra L. leaves obtained from Paser Regency, East Kalimantan, in four different locations, namely Rantau Panjang, Padang Pangrapat, Jone, and Pondong Baru (Figure 1).
Chemicals: The chemicals used included sodium sulfate (Na2SO4) purchased from Merck (Germany), a homologous series of C8–C20 n-alkanes, and DPPH (2,2-diphenyl-1-picrylhydrazyl) from Sigma Chemical Co., while ascorbic acid, ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)), potassium persulfate, methanol, and other chemicals were obtained commercially.
2.1.2. Methods
2.1.2.1. Distillation: Each sample of M. leucadendra L. leaves from the four different locations used approximately 4 kg of air-dried leaves, extracted using water and steam distillation for approximately 4 hours to obtain the essential oil. The essential oil was then stored in airtight containers for testing and labeled according to their plant collection locations: EO1, EO2, EO3, and EO4 for samples from Rantau Panjang, Padang Pangrapat, Jone, and Pondong Baru, respectively.
2.1.2.2. Gas chromatography-mass spectrometry (GC-MS) analysis: The chemical components in the essential oil of M. leucadendra L. were analyzed using gas chromatography-mass spectrometry (GCMS-QP2010 Ultra Shimadzu) according to Kartiko et al. (2021) with slight modifications. The column used was RTX-5MS with a thickness of 0.25 µm, length of 30 m, and inner diameter of 0.25 mm. The gas chromatography system was set with an initial temperature of 70°C, injection temperature of 250°C, temperature increase of 25.00°C/minute to a final temperature of 280°C, column pressure of 93.5 kPa, total flow of 291.5 mL.min–¹, column flow of 1.44 mL.min–¹, flow control mode with linear velocity of 43.9 cm/second, purge flow of 3.0 mL.min–¹, and an injection mode of split with a ratio of 200. Electron ionization mass spectra were recorded over a range of 40–400 m/z. Compound composition was reported as the percentage of peak area (%), after which data were grouped and the main peak components discussed. Retention indices (RI) were calculated using a standard series of n-alkanes C8–C20 under the same conditions. Compounds were identified based on RI values from the literature or the NIST mass spectral library.
2.1.3. Evaluation of antioxidant activity of essential oil
Antioxidant activity was quantified using spectrophotometric assays; DPPH absorbance was measured at 517 nm, while ABTS measurements were conducted at 734 nm, ensuring specificity for radical scavenging activities. DPPH and ABTS were selected for their suitability in evaluating lipophilic antioxidants and their complementary reaction mechanisms
2.1.3.1. DPPH (2,2-diphenyl-1 picrylhydrazyl) assay: The antioxidant activity testing method for DPPH was based on Kuspradini et al. (2020) with slight modifications. Each sample concentration was pipetted in a volume of 33 µL into a test tube, then mixed with 467 µL of methanol and 500 µL of DPPH solution (0.1 mol•L–¹) to obtain final test concentrations of 100 µg•mL–¹, 50 µg•mL–¹, 25 µg•mL–¹, 12.5 µg•mL–¹, and 6.25 µg•mL–¹. The samples were incubated for 20 minutes in a dark room with minimal light at room temperature, then absorbance was measured at 517 nm using a UV-Vis spectrophotometer (model). Ascorbic acid was used as a positive control because it is a well-known natural antioxidant. The spectrophotometer results were recorded to determine the percentage of antioxidant activity.
2.1.3.2. ABTS (2,2 azinobis (3-etilbenzotiazolin)-6-sulfonic acid) assay: The antioxidant testing method using the free radical of ABTS was prepared according to a modified method ( Liu et al., 2022; Toppo et al., 2019; Zhang and Xu, 2015). About 47.5 mg of ABTS was weighed on an analytical scale in a glass beaker, then dissolved in 12.5 mL of methanol to obtain a concentration of 7.4 mmol•L–¹. Subsequently, 4.4 mg of potassium persulfate was weighed and dissolved in 6.25 mL of distilled water to obtain a concentration of 2.6 mM. Both solutions were mixed in a glass beaker at a ratio of 2:1, then incubated for approximately 16 hours at room temperature under minimal light conditions. The incubated ABTS solution was diluted by mixing 1 mL of the solution with 60 mL of methanol to achieve an absorbance of 0.700 ± 0.02 at a wavelength of 734 nm, measured using a spectrophotometer. From each sample stock, 100 µL was placed into a test tube and added to 900 µL of ABTS solution to obtain final test concentrations of 6.25, 12.5, 25, 50, and 100 µg•mL–¹, then mixed for 45 seconds and incubated for 15 minutes in a dark room. Absorbance was measured at 734 nm using a spectrophotometer. Ascorbic acid was used as a positive control, and the results were recorded to determine the percentage of antioxidant activity (Shalaby, 2013).
2.2. Data Analysis
The chemical composition was analyzed using GC-MS and reported as the percentage of peak area (%). The data were then classified into groups, and the main components of the peak compounds were discussed. Retention indices (RI) were calculated using an n-alkanes C8–C20 standard with the same program. Compounds were identified based on the RI values from the literature or the NIST mass spectral library. Each antioxidant analysis was performed in triplicate, and all data were recorded to determine the percentage (%) of antioxidant activity. Antioxidant activity was assessed using DPPH (517 nm) and ABTS (734 nm) assays, both conducted in triplicate. Radical scavenging activity (% inhibition) was calculated as: % Inhibition=[(Acontrol − Asample) / Acontrol] × 100, where Acontrol is the absorbance of the radical solution alone, and Asample AsampleA_{\text{sample}} is the absorbance with the sample.
The IC50, defined as the concentration inhibiting 50% of radicals, was determined by plotting % inhibition against concentration and applying linear regression (y = mx + c) to the linear region, solving for x when y = 50. These metrics—% inhibition and IC50—quantify the essential oil’s antioxidant efficacy and allow for potency comparisons across samples.
3. RESULTS AND DISCUSSION
3.1. Chemical compositions of M. leucadendra oils
The identification results of essential oil constituents in M. leucadendra, obtained through GC-MS analysis, are presented in Table 1.
Table 1
Constituent components of M. leucadendra essential oil from four locations in Paser Regency, East Kalimantan.
[i] Note: Values in bold indicate the main components; - : no compound; RIexp: Retention indices on the RTX-5MS column relative to C8-C20 n-alkanes; RIlit: Retention indices from literature (NIST Chemistry WebBook, SRD 69); Location = EO1 [Rantau Panjang], EO2 [Padang Pangrapat], EO3 [Jone], and EO4 [Pondong Baru].
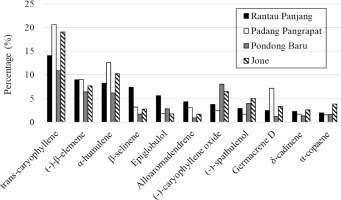
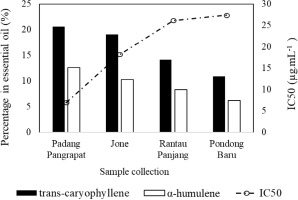
The chemical constituents accounted for 85.89–94.75% of the oils. Based on the results, all analyzed samples contained monoterpenes (1.95–31.87%), sesquiterpenes (56.66–86.57%), and other components (11.45–13.30%). Sesquiterpenes constituted the major group, with hydrocarbon compounds being more abundant than oxidized ones. Only 11 compounds were consistently present in all essential oils, namely trans-caryophyllene, (−)-β-elemene, α-humulene, β-selinene, epiglobulol, aromadendrene, (−)-caryophyllene oxide, (−)-spatulenol, germacrene D, δ-cadinene, and α-copaene; however, only three compounds had concentrations greater than 7% (Figure 2). Figure 3 shows that trans-caryophyllene, (−)-β-elemene, and α-humulene were the major compounds present in M. leucadendra from Paser Regency.
In this study, trans-caryophyllene (TC) was identified as the predominant compound in Melaleuca leucadendra essential oil, with concentrations ranging from 10.87% to 20.62%. Other significant sesquiterpenes included α-humulene, varying between 6.19% (EO3) and 12.57% (EO2), and (−)-β-elemene, present at 6.4% (EO3) to 8.94% (EO1). Notably, the high percentage of TC in this study contrasts with previous reports from other regions, where M. leucadendra essential oil typically contained lower trans-caryophyllene levels, such as 12.5% in Central Kalimantan (Indonesia) (Widiana et al., 2015), 0.1-3.5% in Uttarand (India) (Kholiya et al., 2023), 2.69% in Kerala (India), 2% in Benin, and 3.78%–7.64% in Java (Indonesia) (Adjalian et al., 2015; Sahla and Pushpalatha, 2020; Torry and Idrus, 2016). These differences suggest that both qualitative and quantitative variations in essential oil composition are influenced by multiple factors, including climatic conditions, soil properties, and tree age. Previous studies have shown that as trees mature, β-caryophyllene concentrations increase, whereas 1,8-cineole levels decline (Pujiarti et al., 2011).
Beyond M. leucadendra, other Melaleuca species also exhibit notable variations in essential oil composition, further reinforcing the role of environmental and genetic factors in shaping oil profiles. For instance, M. cajuputi Powell (Malaysia) contains 20.16% caryophyllene, M. styphelioides has 50%, and M. cajuputi from East Java (Indonesia) contains 10.60% (Sharif et al., 2019; Sutrisno et al., 2018). Structurally, trans-caryophyllene, α-humulene, and (−)-β-elemene are sesquiterpenes commonly found as volatile components in aromatic plants, making them valuable in food flavoring, cosmetics, and pharmaceuticals (Wu et al., 2014; Fidyt et al., 2016). These compounds also exhibit distinct sensory properties, with caryophyllene contributing a woody, sweet, and spicy aroma; humulene producing a woody, acidic, and burnt scent; and β-elemene functioning as a key component in floral aromas and insect pheromones (Kiyama et al., 2020). In addition to their industrial applications, these sesquiterpenes possess various pharmacological properties, including anti-inflammatory, antimicrobial, and anticancer effects (Guan et al., 2014; Jang et al., 2020; Zhang et al., 2018).
The chemical composition of Melaleuca essential oils is largely shaped by environmental factors, including soil composition, climate, and geographical location. Soil characteristics such as pH, nutrient content, and organic matter play a crucial role in the biosynthesis of essential oil components. Studies have shown that nitrogen-rich soils promote monoterpene production, while phosphorus and potassium enhance sesquiterpene accumulation (Licata et al., 2015). Additionally, high levels of heavy metals or soil salinity can induce biochemical changes in plants, leading to shifts in terpene profiles (Elshafie and Camele, 2017). In Melaleuca species, variations in soil fertility have been associated with differences in 1,8-cineole, terpinen-4-ol, and trans-caryophyllene concentrations (Pujiarti et al., 2011). Climate conditions—including temperature, rainfall, and seasonal variations—also impact essential oil production. High temperatures increase monoterpene volatilization, resulting in a higher proportion of sesquiterpenes, which are more thermally stable (Wang et al., 2020). Drought stress has been linked to increased biosynthesis of antioxidant-rich secondary metabolites, as plants generate defensive compounds in response to oxidative stress (Floegel et al., 2011). Additionally, seasonal fluctuations affect oil yield and composition, with dry-season harvests generally containing higher concentrations of bioactive terpenes than wet-season samples (Sharif et al., 2019).
The geographical location of Melaleuca plants, including altitude, latitude, and proximity to coastal or inland environments, further influences terpene biosynthesis. High-altitude regions tend to favor the production of oxygenated terpenes, such as caryophyllene oxide and viridiflorol, due to greater UV exposure and oxidative stress (Guerrini et al., 2009). Conversely, coastal environments, with their high humidity and saline conditions, impact oil composition by affecting water absorption and nutrient uptake (Torres-Martínez et al., 2017). Moreover, chemotypic diversity has been observed within the same Melaleuca species, where microclimatic variations lead to distinct essential oil compositions, even among plants from the same region (Siddique et al., 2020)
3.2. Antioxidant activities of M. leucadendra essential oil
In vitro, antioxidant assays are designed to simulate the oxidation-reduction reactions commonly occurring in living biological systems and are widely used to estimate the antioxidant potential of various chemical and biological samples. In this study, two complementary assays—DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2'-azinobis(3-ethylbenzothiazolin-6-sulfonic acid))—were employed to evaluate the antioxidant activity of Melaleuca leucadendra essential oils collected from different locations.
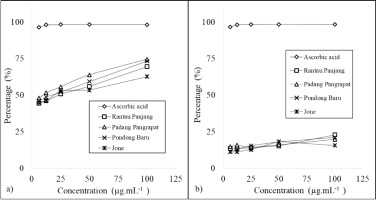
Based on the results presented in Figure 4, the percentage inhibition of the essential oils was concentration-dependent. The oils exhibited ABTS radical scavenging activity ranging from 44.1% to 74.5% at concentrations between 6.25 and 100 µg•mL–¹, whereas the DPPH assay showed a lower inhibition range of 7.76% to 18.17%. These differences in antioxidant activity likely reflect the complexity, polarity, and chemical characteristics of the essential oils, which can influence assay outcomes depending on the method used (Guerrini et al., 2009). Additionally, environmental factors such as plant origin and climatic conditions affect the chemical composition of the oils, thereby contributing to variability in their antioxidant properties (Elshafie and Camele, 2017).
The antioxidant activities of each essential oil, assessed by the DPPH and ABTS assays, were expressed as the half-maximal inhibitory concentration (IC50) — the concentration required to inhibit 50% of the free radicals. A lower IC50 value indicates stronger antioxidant activity. The IC50 values, calculated from linear regression analysis of antioxidant activity versus concentration, were within the µg.mL-1 range for all samples. There were notable differences between the antioxidant activities measured by the DPPH and ABTS assays. M. leucadendra essential oils exhibited strong antioxidant activity in the ABTS assay, with IC50 values ranging from 6.95 to 26.14 µg•mL–¹, whereas the DPPH assay indicated weak antioxidant activity, with IC50 values between 375 and 701.21 µg•mL–¹. According to Phongpaichit (2007), IC50 values of 10–50 µg•mL–¹, 50–100 µg•mL–¹, and above 100 µg•mL–¹ correspond to strong, intermediate, and weak antioxidant activity, respectively. Similarly, Blois (1958) classified antioxidants with IC50 values below 50 µg•mL–¹ as very strong, 50–100 µg•mL–¹ as strong, 101–150 µg•mL–¹ as medium, and greater than 150 µg•mL–¹ as weak (Blois, 1958).
The antioxidant activity of M. leucadendra oil in the ABTS assay can be classified as very strong, as it has an IC50 value lower than 50 µg•mL–¹. In contrast, the sample is considered to exhibit weak activity based on the DPPH assay. Similar findings have been reported in several essential oils that show higher ABTS radical scavenging activity compared to DPPH results (Silva et al., 2018; Fathollahi et al., 2019; Ferreira et al., 2021). There is often a weak correlation between DPPH and ABTS results, as some compounds that display ABTS radical scavenging activity may not exhibit significant activity in the DPPH assay when tested in essential oils or plant extracts (Liu et al., 2022; Sharopov et al., 2015). Despite this, the DPPH method offers several advantages over other antioxidant assays. These include its convenience, affordability, widespread use in in vitro analyses, simplicity, speed, low sample requirement, minimal reagent consumption, and compatibility with a wide range of solvents, from nonpolar to polar types (Akar et al., 2017).
The ABTS method has higher sensitivity than DPPH and is commonly employed to analyze oxidants in food. Both methods operate through distinct reaction mechanisms: DPPH assesses antioxidant activity based on hydrogen atom donation, whereas ABTS evaluates a compound’s ability to stabilize free radicals through proton donation. Despite these differences, both assays rely on hydrogen atom abstraction, in which antioxidants react with DPPH• or ABTS•+ free radicals to form alkyl, alkoxy, or aryloxy radicals. Consequently, the radical scavenging ability of essential oil components may be associated with their bond dissociation energy (Yan-Hwa et al., 2000).
ABTS dissolves in both organic solvents and water, enabling it to detect both lipophilic and hydrophilic compounds. However, it does not accurately reflect the body's biological defense system against free radicals and is therefore more suitable as a comparative assay (Karadag et al., 2009; Andrade et al., 2013). In contrast, DPPH is soluble only in organic solvents, making it less effective in interacting with highly lipophilic components such as M. leucadendra essential oil, which is predominantly composed of non-polar sesquiterpenes like trans-caryophyllene and α-humulene (Chadwick et al., 2013; Sut et al., 2018). Consequently, the DPPH assay yielded lower antioxidant activity for M. leucadendra oil compared to ABTS. This finding aligns with previous studies reporting that essential oils rich in sesquiterpene hydrocarbons exhibit greater ABTS radical scavenging activity due to increased solubility and reactivity in ABTS assays (Ehsani et al., 2016; Sitarek et al., 2017; Chadwick et al., 2013; Sut et al., 2018).
Floegel et al. (2011), suggest that the ABTS assay is more effective than DPPH for evaluating antioxidant capacity across various food samples, as it better accounts for factors such as moisture content, pigmentation, and the presence of both lipophilic and hydrophilic compounds. Additionally, the ABTS method is known for its high reproducibility, offering greater sensitivity and consistency compared to the DPPH assay (Martysiak-Żurowska and Wenta, 2012; Sadeer et al., 2020). Most natural antioxidants function synergistically, providing a broad spectrum of activity that supports an effective defense system against free radical incursion (Ruberto and Baratta, 2000). Therefore, the essential oil from M. leucadendra L. may be utilized as a natural antioxidant. However, the ABTS method should not be regarded as superior to DPPH in all contexts, as it does not accurately represent the body's endogenous defense mechanisms and is primarily used for evaluating antioxidant capacity in foods, beverages, and related substances (Floegel et al., 2011; Karadag, et al., 2009). While DPPH and ABTS assays are widely employed for assessing the antioxidant potential of natural products, each has inherent limitations that may not fully capture the complexity of antioxidant mechanisms in essential oils. Consequently, future studies should consider incorporating additional assays, such as Ferric Reducing Antioxidant Power (FRAP), Oxygen Radical Absorbance Capacity (ORAC), and lipid peroxidation inhibition assays, to provide a more comprehensive evaluation of the antioxidant pathways involved in M. leucadendra essential oil (Gulcin, 2020; Prior et al., 2005).
The ABTS radical scavenging activity of M. leucadendra essential oils followed the order: Padang Pangrapat > Pondong Baru > Rantau Panjang > Jone. A lower IC50 value indicates stronger antioxidant activity. This antioxidant activity trend aligns with the concentration of major constituents in the essential oils, particularly trans-caryophyllene and α-humulene (Figure 5). Essential oils containing specific active components, particularly trans-caryophyllene, have been reported to exhibit significant antioxidant activity. Trans-caryophyllene often emerges as the predominant contributor to radical scavenging effects in such oils. Additionally, the presence of α-humulene may enhance the overall antioxidant potential, possibly through synergistic interactions. Notably, α-humulene has also been shown to possess anti-inflammatory properties, further supporting its therapeutic value (Legault and Pichette, 2010).
Trans-caryophyllene, the principal isomer of β-caryophyllene (BCP), naturally coexists with minor isomers such as (Z)-β-caryophyllene (iso-caryophyllene), α-humulene (also known as α-caryophyllene), and its oxidized form, β-caryophyllene oxide. Despite its low solubility in water—which limits absorption in aqueous biological environments—trans-caryophyllene has demonstrated a strong affinity for lipid bilayers, indicating its potential to interact with and penetrate cellular membranes (Fidyt et al., 2016; Sarpietro et al., 2015). To overcome the solubility barrier and improve bioavailability, liposomal drug delivery systems have been proposed as effective carriers. Comprehensive toxicological assessments have shown that BCP is non-genotoxic, non-phototoxic, and free of reproductive toxicity risks, as documented by the International Fragrance Association (IFRA) Standards. Furthermore, environmental risk analyses classify BCP as non-persistent, non-bioaccumulative, and non-toxic (non-PBT), supporting its safe application in various industries. The U.S. Food and Drug Administration (FDA) has also designated BCP as “Generally Recognized As Safe” (GRAS) for use as a flavoring agent and additive in food and cosmetics (CFR Title 21, 2020). Given its efficient metabolism, antioxidant capacity, and minimal ecological footprint, trans-caryophyllene presents strong potential for expanded pharmaceutical, nutraceutical, and commercial applications (Api et al., 2022).
4. CONCLUSION
The results of this study demonstrate consistent chemical profiles and antioxidant activities among essential oils extracted from Melaleuca leucadendra collected across different locations in Paser Regency. All four essential oil samples were dominated by the sesquiterpene hydrocarbons trans-caryophyllene, α-humulene, and (–)-β-elemene, with eleven compounds consistently present in all samples: trans-caryophyllene, (–)-β-elemene, α-humulene, β-selinene, epiglobulol, aromadendrene, (–)-caryophyllene oxide, (–)-spathulenol, germacrene D, δ-cadinene, and α-copaene. Among the antioxidant evaluations, the ABTS assay revealed strong radical scavenging activity, ranging from 44.1% to 74.5%, with IC50 values below 50 µg•mL–¹—classifying the oils as very strong antioxidants. Notably, the antioxidant activity showed a positive correlation with the levels of trans-caryophyllene and α-humulene, suggesting these compounds play a significant role in the bioactivity of the oils. Given the ABTS assay’s higher sensitivity and its relevance for food antioxidant evaluation, M. leucadendra essential oil shows strong potential as a natural preservative against oxidative stress in food products. However, while this study offers promising insights, it is primarily limited to in vitro radical scavenging assays (DPPH and ABTS). For a more comprehensive assessment, future studies should integrate additional analytical methods such as FRAP, ORAC, and lipid peroxidation inhibition assays. Furthermore, in vivo studies are essential to evaluate bioavailability, metabolic stability, and therapeutic efficacy. Investigations into toxicity, safety, and the influence of environmental variables on oil composition will be critical for advancing the pharmaceutical and commercial viability of M. leucadendra essential oils.
ACKNOWLEDGEMENTS
The authors would like to thank The Forestry Faculty of Mulawarman University and The Research Center for Medicine and Cosmetics from Tropical Rainforest (PUI-OKTAL) Universitas Mulawarman, East Kalimantan, Indonesia for financial support in part of this research.
AUTHOR CONTRIBUTIONS
H.K.: Research concept and design, Collection and/or assembly of data, Writing the article, Final approval of the article; A.B.B.K.: Collection and/or assembly of data; A.S.P.: Data analysis and interpretation; W.S.: Critical revision of the article; E.R.: Critical revision of the article.