1. INTRODUCTION
Breast cancer remains a major contributor to cancer-related deaths worldwide. According to GLOBOCAN 2020, breast cancer accounts for ~11.7% of all cancer cases globally, while 2.3 million new cases and 670,000 deaths from female breast cancer were recorded in 2022 (Kim et al., 2025; Sung et al., 2021). In Indonesia, among a total of 396,914 newly diagnosed cancer cases, 68,858 were new breast cancer cases (16.6%). Additionally, more than 22,000 (9.6%) breast cancer-related deaths were recorded, with an average mortality rate of 17 per 100,000 individuals (Ng et al., 2023; “The Global Cancer Observatory,” 2020). Forecasts indicate that by the year 2050, the number of new breast cancer cases and associated deaths will rise by approximately 38% and 68%, respectively, with the sharpest increases expected in countries with lower Human Development Index values (Kim et al., 2025). These findings highlight the increasing burden of breast cancer, and therefore, the identification of natural compounds with potential therapeutic benefits is of great importance.
Extensive research has been conducted to uncover the fundamental mechanisms of cancer and identify innovative and effective therapeutic approaches. One such natural substance, Holothuroidea, is a sea cucumber, which is traditionally used in folk medicine due to its bioactive compounds. Sea cucumbers, also known as “hai shen” or “sea ginseng” have been widely used for centuries across Asia, especially in China, as both a nutritional delicacy and a medicinal tonic (Chen, 2003). Sea cucumber is believed to nourish the kidneys, fortify yang energy, and promote wound healing, vitality, and longevity. Recent scientific research has revealed that sea cucumbers are rich in bioactive constituents, such as triterpene glycosides (holothurins), peptides, sulfated polysaccharides, and sterols, which play a role as antioxidant, anti-inflammatory, immunomodulatory, and anticancer activities (Hossain et al., 2022; Janakiram et al., 2015; Wargasetia et al., 2023).
Among these, Holothuria scabra, a widely cultivated species in Southeast Asia, has attracted attention for its high content of bioactive saponins, known as holothurins, which have shown potential anticancer activity in preclinical studies (Khotimchenko, 2018). These bioactive compounds may exert their effects by inhibiting cell growth, triggering programmed cell death, and preventing the spread of cancer cells (Sajwani, 2019). Previous studies have demonstrated that metabolites from sea cucumbers can interfere with tumor progression by modulating key cellular signaling pathways, including PI3K/AKT and TGF-β (Ratnawati et al., 2024).
Recent studies have increasingly focused on combining in silico methods with experimental approaches to gain deeper insights into the molecular mechanisms underlying the anticancer activity of natural compounds. Bioinformatics tools are widely used to discover promising medical treatment agents for a range of diseases. Structure-based drug design supports this process by enabling virtual screening, which assesses how well candidate compounds interact with specific protein receptors through binding affinity evaluations, thus allowing researchers to identify promising therapeutic candidates prior to conducting in vitro and in vivo experiments (Anandan et al., 2023; Marin et al., 2023; Samadarsi et al., 2022). In silico methods, such as molecular docking, can predict the interactions between bioactive compounds and specific cellular targets (Stanzione et al., 2021; Yang et al., 2022). The present study aimed to investigate the interaction between Holothuria scabra methanol extract (HSE) with proteins involved in apoptosis regulation, a crucial process in cancer progression. Specifically, molecular docking was used to explore the holothurin’s potential interaction with the proapoptotic BAX protein and the antiapoptotic BCL-2 protein. These two proteins regulate apoptosis in opposing ways, and disruptions in their balance are known to significantly influence the development and progression of cancer cells (Alberts B., 2022; Qian et al., 2022).
In silico approaches can help uncover potential interactions between holothurin and key proteins that play a central role in the apoptotic pathway (Wargasetia et al., 2020). Following the in silico analysis, in vivo experiments were performed using a breast cancer animal model to assess the possible therapeutic potential of HSE. This current research aimed to determine whether treatment with HSE could modulate the expression of apoptosis-related genes. Specifically, the study mainly focused on evaluating the ability of HSE to upregulate Bax and downregulate Bcl-2 in breast cancer tissues, ultimately restoring the apoptotic process and reducing tumour growth (Aminin et al., 2015; Assawasuparerk et al., 2016; Qian et al., 2022).
A previous in silico study revealed that particular sea cucumber peptides could bind to phosphoinositide 3-kinase (PI3K) and protein kinase B (AKT1), and the results showed a stable interaction, but low binding affinity (Wargasetia et al., 2021). These findings suggested that these peptides could exert strong potential as antibreast cancer agents, thus prompting us to continue with in vivo study using a breast cancer mouse model. An in vivo study further demonstrated that HSE inhibited cancer progression via the TGF-β/PI3K/phosphatase and tensin homolog signaling pathway (Ratnawati et al., 2024). Given that carcinogenesis is a complex process involving multiple genes and signaling pathways, further studies are needed to explore the effect of Holothuria scabra on inducing cell apoptosis in breast cancer. Previous studies also indicated that holothurin could act as a tumor suppressor and enhance apoptosis (Assawasuparerk et al., 2016; Bahrami et al., 2016; Ru et al., 2022).
Given the historical use of sea cucumbers in traditional medicine and their emerging scientific relevance, Holothuria scabra represents a promising source of therapeutic agents from nature. In this context, this study aimed to explore its anticancer potential through the apoptosis pathway more systematically using modern bioinformatics and experimental tools as new treatment strategies for breast cancer.
2. MATERIALS AND METHODS
2.1. In Silico Study
Molecular docking was employed to conduct the in silico study, a computer-aided drug discovery technique that functions through a structure-based drug design approach, to predict the interaction between ligands and target receptors (Agu et al., 2023; Yang et al., 2022). The molecular docking technique was applied to estimate both the interactions and binding affinities between receptors (Bax and Bcl-2) and ligands, namely holothurin A (PubchemID, 23675050; MW, 1221.3 g/mol), holothurin B (PubchemID, 23674754; MW, 883.0 g/mol), and holothurin B3. Since the 2D structure of holothurin B3 was not found, its structure was modified from holothurin B according to the article by Claereboudt et al (Claereboudt et al., 2023; Guedes et al., 2014). Docking evaluation was performed based on the scores obtained using AutoDock4.2 (AV). The best conformation of the holothurin-receptor complex was determined based on the lowest binding free energy.
2.2. Receptor Preparation
The 3D structures of Bax (6O0K) and Bcl-2 (4BD6) receptors were downloaded from the Protein Data Bank (http://www.rcsb.org/pdb) in .pdb format. The files were opened with AutodockTools version 1.5.7. Water molecules and any ligands bound to the receptors were removed from the structure, while polar hydrogen atoms were added to the receptors. Finally, the files were saved in .pdbqt format.
2.3. Receptor-Ligand Docking
The receptor-ligand docking was performed using Autodock4 in Windows with the default configurations and according to the protocol reported by Forli et al. (2016). Docking was carried out within each protein’s binding pocket at the location of the crystallized ligand. The docking grid search space was defined with dimensions of 40 × 40 × 40 and centered on the crystallized ligand’s location. Since there was no crystallized ligand for Bax protein, the binding site was predicted using the https://prankweb.cz/ website. The highest-likelihood binding site was selected for docking. The ligand was docked using the genetic algorithm method for 50 iterations.
2.4. Validation of Receptor-Ligand Complex Structure
To determine whether the docked ligand could bind at a location similar to its cocrystal ligand, the protocol was validated via comparing the conformation of the redocked crystallized ligands with their original conformation. From molecular docking, the root mean square deviation (RMSD) was obtained, a value that is calculated based on the atomic distance of the docked ligand to the coordinates of its cocrystal ligand. A lower RMSD value indicated a closer match between the redocking result and the cocrystal ligand’s pose. An RMSD value of ≤2.0 Å signified a good redocking quality, while an RMSD value of 2-3 Å fell into the acceptable quality category. Finally, an RMSD value of ≥3.0 Å indicated poor quality (Ramírez and Caballero, 2018). Therefore, herein, an RMSD of <2 Å was used as the cutoff to validate the docking protocol. However, redocking validation was not performed for the Bax protein due to the limited number of reported small-molecule inhibitors or activators in the current literature. Bax predominantly regulates apoptosis through interactions between proteins with other members of the Bcl-2 family, rather than via direct binding with small molecules. Therefore, molecular docking was employed as one of the methods that can be chosen to predict potential binding sites and interactions.
2.5. Holothuria scabra Methanol Extraction
Fresh Holothuria scabra specimens were collected from Malang Coastal, East Java, Indonesia, and identified according to the Food and Agriculture Organization (FAO) Species Catalog (Pangestuti and Arifin, 2018). Subsequently, a total of 20 g of sea cucumber was finely ground into powder and subjected to extraction with 500 mL of absolute methanol through a 48-hour maceration process. The resulting mixture was filtered using Whatman No. 41 filter paper and then evaporated at 50-60°C using a Buchi R-114 rotary evaporator.
2.6. Animal Model Establishment and Experimental Design
This study has received ethical approval from the official Research Ethics Committee, and all procedures were performed in accordance with applicable national regulations and institutional standards for laboratory animal care and use.
A total of 30 female C57BL/6 mice (Mus musculus; age, 10-11 weeks) were obtained from iRATCo Veterinary Laboratory Services, Bogor, Indonesia (Hiraga and Ninomiya, 2019). All procedures were performed in accordance with applicable national regulations and institutional standards for laboratory animal care and use. Prior to the initiation of the experiment, female Mus musculus were acclimatized for 1 week, then randomly divided into the following five groups (n = 6 mice per group): i. The negative control (NC) group, consisting of healthy mice fed with a standard diet; ii. the positive control (PC) group, including mice with breast cancer; and iii. the treatment groups (T1, T2, T3), comprising mice with breast cancer treated with different HSE doses. The breast cancer in vivo mouse model was established via a combination of high-fat diet (HFD) and chemical induction with 1 mg/kg body weight (BW) 7,12-dimethylbenz[a]anthracene (DMBA; cat. no. D3254; Sigma-Aldrich; Merck KGaA) dissolved in sesame oil (Plante, 2021). To promote tumor development, all mice, except those in the NC group, were fed an HFD (57% fat) twice daily for 21 days. On day 22, 0.5 mL DMBA was administered subcutaneously into the right mammary tissue, with repeated doses every 2 days for a total of 10 administrations (Cranford et al., 2019; Abba et al., 2016; Thompson and Singh, 2000; Z’graggen et al., 2001). Mice in the treatment groups (T1, T2, and T3) received different doses of Holothuria scabra extract (0.33, 0.66, and 0.99 g/kg BW, respectively) via oral gavage once daily. HSE administration commenced on day 22, alongside the first DMBA injection, and continued for 8 weeks (Cahyati et al., 2018).
Tumor palpation was performed once per week until a tumor was detectable. Once palpable, tumor documentation was performed every 3 days, including BW, tumor size (measured with calipers), general condition, and mice activity recordings. To ensure ethical treatment and animal welfare, all mice were monitored daily for general health, behavior, and signs of pain or distress. At the end of the study, the average circular tumor area in the PC group was 194.64 ± 17.07 mm2, while no tumors were recorded in the NC group (Pranoto et al., 2023). At the end of the experiment, the mice were anesthetized by intraperitoneal injection of ketamine HCl (100 mg/kg BW) combined with xylazine (10 mg/kg BW), and subsequently euthanized through cervical dislocation. The breast tissues were then excised, weighed, and analyzed histopathologically using the Nottingham scoring system to assess tumor development and malignancy (Wargasetia et al., 2024). The remaining mammary gland tissues were stored at −80°C for further molecular analysis.
2.7. RT-qPCR Was Used to Analyze Gene Expression
Total RNA was extracted from mammary gland tissues using the Genezol™ kit (Geneaid Biotech Ltd.) following the manufacturer’s protocol and reverse transcribed into cDNA with the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Inc.). Bax and Bcl-2 mRNA levels were quantified using Thunderbird SYBR qPCR Mix (Toyobo Co., Ltd.), with GAPDH as the internal control (Ghatei et al., 2017). Primer sequences for BAX, Bcl-2, and GAPDH are shown in Table 1.
Table 1
Primer sequences of BAX, Bcl-2, and GAPDH genes
Each qRT-PCR mixture consisted of 3 μL of cDNA, 0.5 μL of SYBR Green Master Mix, 0.6 μL of forward and reverse primers, and PCR-grade water to reach a total volume of 20 μL. The thermocycling program included an initial denaturation at 94°C for 2 minutes, followed by 30 cycles of denaturation at 94°C for 10 seconds, annealing at 60°C for 20 seconds, and extension at 72°C for 70 seconds, with a final hold at 4°C. All reactions were carried out in triplicate. The expression levels of the apoptosis-related genes BAX and Bcl-2 were determined using a relative quantification approach. Relative quantification allows for comparing gene expression between different experimental groups by normalizing to an internal reference gene and a control condition. This method enables us to observe how treatment modulates the expression of apoptosis-related genes in comparison to untreated cancer conditions (Livak and Schmittgen, 2001).
2.8. Statistical Analysis
Statistical analyses were performed using SPSS version 25.0 (IBM Corp.). Normally distributed data were analyzed by one-way ANOVA with Tukey’s HSD post hoc test, while non-normally distributed data were assessed using the Kruskal-Wallis test followed by the Mann-Whitney test. Differences were considered statistically significant at p ≤ 0.05.
2. RESULTS AND DISCUSSION
Breast cancer continues to be among the most common and deadly types of cancer worldwide. The main approach to treatment typically involves surgical removal of the tumor, which is frequently accompanied by additional therapies like chemotherapy and radiation therapy. While these adjuvant treatments improve patient outcomes, they are frequently associated with significant side effects and the emergence of resistance, thereby limiting their long-term efficacy. Therefore, there is a pressing need to explore natural therapeutic agents with minimal adverse effects that can function as adjuvant therapies, helping to reduce recurrence and improve patient survival rates (Liao et al., 2013).
The current study investigated the methanol extract of Holothuria scabra (HSE) as a potential natural therapeutic agent for breast cancer through an integration of in silico and in vivo studies. H. scabra has long been used in traditional medicine throughout Asia and the Middle East, largely due to its bioactive compounds such as holothurin (Bahrami et al., 2016; Hossain et al., 2022)
3.1. In silico Study
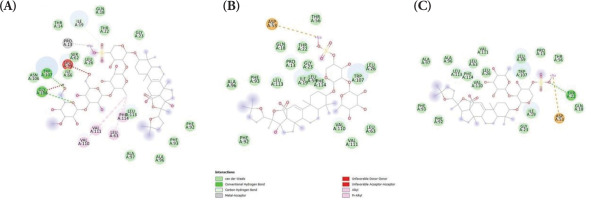
Firstly, the binding affinities between the holothurin compounds, holothurin A, B and B3, and two essential proteins associated with apoptosis regulation, namely Bax and Bcl-2, were evaluated. Since the protein structure of Bax with a cocrystallized ligand could not be found due to the lack of reported small-molecule inhibitors or activators in the literature, the binding capacity was predicted using molecular docking. Emerging evidence has suggested that BAX predominantly regulates apoptosis via protein-protein interactions with other members of the Bcl-2 family but not via directly interacting with small molecules (Ghatei et al., 2017; Ramírez and Caballero, 2018). Since these natural ligands are proteins, they were unsuitable for the current docking study, which mainly focused on small-molecule interactions. Therefore, molecular docking was employed as an alternative approach to predict potential binding sites and interactions. Herein, several pockets of possible docking sites were identified and the one with the highest binding probability (probability, 0.267; coordinates, 5.19, −30.54, and 0.44) was selected. The binding energies between Bax and holothurin A (ΔG = −8.325 kcal/mol), Bax and holothurin B (ΔG = −7.896 kcal/mol), and Bax and holothurin B3 (ΔG = −8.036 kcal/mol) are illustrated in Figure 1, respectively.
Figure 1
The binding affinity of BAX protein with (A) Holothurin A (ΔG = −8.325 kcal/mol); (B) Holothurin B (ΔG = −7.896 kcal/mol); (C) Holothurin B3 (ΔG = −8.036 kcal/mol).
Furthermore, the binding of holothurins on Bcl-2 protein displayed moderate binding affinity. However, the binding affinity of holothurins with Bcl-2 was much less compared with that predicted between the control cocrystallized ligand venetoclax and Bcl-2 (ΔG =−12.138 kcal/mol). Consistently, the binding energies between Bcl-2 and holothurin A (ΔG = −8.732 kcal/mol), holothurin B (ΔG = −7.757 kcal/mol), and holothurin B3 (ΔG = −8.356 kcal/mol) are shown in Figure 2.
Figure 2
The binding affinity of BCL-2 protein with (A) Holothurin A (ΔG = −8.732 kcal/mol); (B) Holothurin B (ΔG = −7.757 kcal/mol); (C) Holothurin B3 (ΔG = −8.356 kcal/mol).
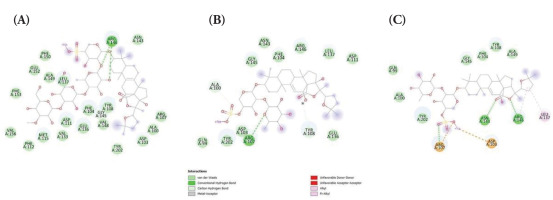
As expected, the control ligand (venetoclax) demonstrated a more negative binding energy, indicating stronger binding affinity, which reflects its rational optimization as a clinically developed Bcl-2 inhibitor. In contrast, holothurins are naturally occurring compounds and have not undergone structure-based optimization. Nevertheless, their moderate binding affinities suggest a potential ability to interact with Bcl-2 and Bax, supporting their prospective role in Bcl-2 inhibition and Bax activation. These findings indicate that holothurins may serve as promising lead compounds, warranting further experimental validation.
Our in silico molecular docking analysis revealed that holothurin has the ability to bind with Bax and Bcl-2 receptors, which are key regulators of the mitochondrial (intrinsic) apoptosis pathway. This computational finding suggests that holothurin may exhibit its anticancer effects through modulation of apoptotic signaling. To further ascertain the potential effects of these compounds, more studies are needed. The combination of computational predictions with in vivo validation, as performed in this study, represents a rational approach to natural compound-based drug discovery and may accelerate the development of novel marine-derived anticancer agents.
3.2. In vivo Study
In the present study, DMBA, a polycyclic aromatic hydrocarbon, was employed as a chemical carcinogen to induce breast cancer in animal models via causing DNA mutations, thus mimicking breast cancer initiation and progression. This approach allows the investigation of tumor biology and the evaluation of potential therapeutic interventions (Abba et al., 2016). The study examined the effects of HSE with three different doses [0.33 (T1), 0.66 (T2), and 0.99 g/kg BW (T3)] on breast cancer in an in vivo mouse model. To assess tumor development, breast tumor weight and Nottingham histological scores were used. The results of the above markers verified the development of breast cancer in the animal mouse model (Pranoto et al., 2023). After killing the animals, RNA was isolated from mammary tissue samples to evaluate the expression of the apoptosis-associated genes Bax and Bcl-2 through RT-qPCR analysis.
3.3. HSE Upregulates Bax
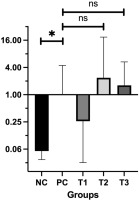
Bax, located on chromosome 19q13.33, plays a pivotal role in promoting apoptosis, specifically through the intrinsic mitochondrial pathway. Bax facilitates apoptosis via promoting the release of cytochrome C from mitochondria, which in turn triggers the caspase cascade, responsible for programmed cell death. This mechanism serves a key role in eliminating potentially cancerous cells (Cavallo et al., 2011). The relative Bax mRNA expression levels in the treatment groups (T1, T2, T3) compared with the PC group are shown in Figure 3.
The findings revealed a significant difference in Bax expression between the PC group (breast cancer model) and the NC group (P = 0.05). Among the treatment groups, Bax expression levels were elevated in T2 (2.36 ± 0.13) and T3 (1.61 ± 0.30) compared to the PC group, while T1 showed a lower expression level (0.26 ± 0.12). These results indicate that administering HSE at doses of 0.66 and 0.99 g/kg BW may enhance Bax expression. Normality and homogeneity of the data were confirmed using the Shapiro-Wilk test (P > 0.05), validating the use of one-way ANOVA followed by Tukey’s HSD post-hoc test. However, post hoc analysis showed no statistically significant differences in Bax expression between the PC group and either T2 (P = 0.911) or T3 (P = 0.989), suggesting that while there was an upregulation of Bax, the increase was not statistically significant (P > 0.05).
3.2. HSE Downregulates Bcl-2
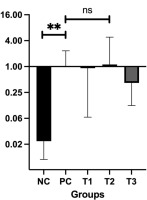
Bcl-2 functions as an antiapoptotic protein that counterbalances Bax by regulating the permeability of the mitochondrial outer membrane and preventing apoptosis through the inhibition of cytochrome C release. Its main role is to support cell survival by binding to and neutralizing proapoptotic proteins such as Bax and Bak (Hanahan and Weinberg, 2011). Figure 4 presents the relative mRNA expression levels of Bcl-2.
Therefore, the results showed that the treatment of mice with various doses of HSE reduced the relative expression levels of Bcl-2. However, no statistically significant difference was observed compared with the PC group (breast cancer mice model). The relative expression levels of Bcl-2 were compared between the NC and treatment groups and the PC group. The results showed a significant difference in Bcl-2 expression levels between the NC and PC groups (P=0.002), ascertain that Bcl-2 expression is highly significantly different between cancer and normal tissue. In addition, HSE treatment reduced the mRNA Bcl-2 levels in T1 (0.92 ± 0.19), T2 (0.87 ± 0.21), and T3 (0.46 ± 0.41) groups compared with the PC group (1.00 ± 0.18). The reduction in Bcl-2 expression appeared to be dose-dependent, with higher HSE doses leading to a more robust reduction in Bcl-2 expression. Statistical analysis using one-way ANOVA with Tukey’s HSD post hoc test indicated no statistically significant differences between the PC group and any of the treatment groups (T1: P = 1.000; T2: P = 1.000; T3: P = 0.898). Therefore, although a downward trend in Bcl-2 gene expression was observed following HSE treatment, the change was not statistically significant (P > 0.05). Consequently, Bcl-2 expression levels were not directly compared between the treatment groups (T1, T2, T3) and the PC or NC groups. Given that Bax and Bcl-2 expression levels differed markedly between normal and cancerous tissues, such direct comparisons would not have been meaningful.
3.5. HSE Increases the Bax/Bcl-2 Ratio
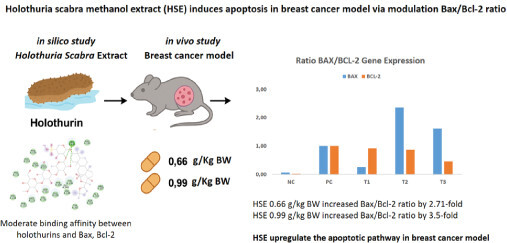
The Bax/Bcl-2 ratio serves as a key indicator for evaluating the equilibrium between pro-apoptotic and antiapoptotic signals in cells, which is critical for determining cell survival or death. In this study, the Bax/Bcl-2 ratio was calculated for all experimental groups, as shown in Table 2.
Table 2
The Bax/Bcl-2 Ratio.
| Treatment Groups | Bax Gene | Bcl-2 Gene | Bax/Bcl-2 Ratio |
|---|---|---|---|
| NC | 0.06 ± 0.39 | 0.02 ± 0.38 | 3.00 |
| PC | 1.06 ± 0.01 | 3.27 ± 0.04 | 0.32 |
| T1 | 0.26 ± 0.12 | 0.92 ± 0.07 | 0.28 |
| T2 | 2.36 ± 0.13 | 0.87 ± 0.23 | 2.71 |
| T3 | 1.61 ± 0.30 | 0.46 ± 0.33 | 3.50 |
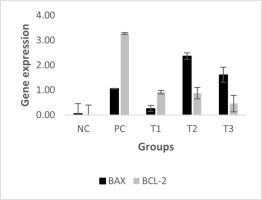
The BAX/Bcl-2 ratio reflects the balance between proapoptotic (BAX) and anti-apoptotic (Bcl-2) signaling within cells. A ratio >1 indicates a shift toward apoptosis, while a ratio <1 suggests dominance of antiapoptotic activity. The positive control (PC) group showed a reduced BAX/Bcl-2 ratio (ratio BAX/Bcl-2 = 0.32), indicating that the activity of Bcl-2 exceeded that of BAX, or the cancer cell continued proliferation, a hallmark characteristic of cancer. Treatment with 0.66 and 0.99 g/kg BW HSE (T2 and T3) increased the BAX/Bcl-2 ratio >1, suggesting enhanced susceptibility to apoptosis. Figure 5 shows the ratio between BAX and Bcl-2 expression for each group.
Figure 5 illustrates the mRNA expression of BAX, a pro-apoptotic gene, and Bcl-2, which has anti-apoptotic properties. This figure also depicts the BAX/Bcl-2 ratio, a commonly used molecular marker to assess the apoptotic potential in cancer studies. BAX and Bcl-2 are central regulators in the intrinsic (mitochondrial) apoptotic pathway. BAX facilitates apoptosis by disrupting mitochondrial membrane integrity, whereas Bcl-2 prevents it by maintaining mitochondrial membrane stability (Alberts, 2022; Qian et al., 2022). As such, the balance reflected by the BAX/Bcl-2 ratio is vital for determining whether a cell will persist or undergo apoptosis. In our study, we used this ratio to assess the proapoptotic versus anti-apoptotic balance in the mammary tissues of breast cancer-induced mice following treatment with different doses of Holothuria scabra extract (HSE):
In the positive control (PC) group, the BAX/Bcl-2 ratio was 0.32, indicating a dominance of antiapoptotic activity, which supports the cancer cells to survive and proliferate. The T1 group (0.33 g/kg BW HSE) showed a similar pattern (ratio = 0.28), suggesting no significant proapoptotic shift. However, in the T2 and T3 groups (0.66 and 0.99 g/kg BW HSE), the ratio increased by ~2.7- and 3.5-fold, respectively, reaching values >1.0. This indicates a shift toward increased apoptotic susceptibility, which is desirable in anticancer therapy, and serves as a functional marker for evaluating the pro-apoptotic efficacy of the treatment, thus supporting that the aforementioned HSE doses could promote cancer cell apoptosis and potentially increase their sensitivity to treatments inducing cell death.
Our results support the hypothesis that HSE modulates apoptosis by upregulating the Bax/Bcl-2 ratio. Although the observed changes in expression levels were not statistically significant (p > 0.05), there was a clear dose-dependent trend. Notably, the Bax/Bcl-2 ratio, a key determinant of a cell’s susceptibility to apoptosis, increased in mice treated with higher doses of HSE. The ratio in the T2 group (0.66 g/kg BW) was 2.76, and in the T3 group (0.99 g/kg BW) it reached 3.5, compared to only 0.34 in the untreated positive control group (PC). A Bax/Bcl-2 ratio greater than 1.00 is widely regarded as an indicator of pro-apoptotic conditions, suggesting that HSE at these doses promotes apoptotic activity in breast cancer tissues.
Apoptosis plays a crucial role in preserving tissue balance and removing cells that are damaged or abnormal, including malignant cells. Within the Bcl-2 protein family, Bax and Bcl-2 are key regulators of the intrinsic apoptosis pathway. Bax acts as a proapoptotic molecule that responds to stressors like DNA damage by moving to the mitochondrial outer membrane, where it oligomerizes and promotes membrane permeabilization to initiate cell death. This process results in the release of cytochrome c and subsequent activation of caspases, leading to programmed cell death. In contrast, Bcl-2 is an antiapoptotic protein that inhibits apoptosis by binding to and neutralizing Bax and other proapoptotic factors, thereby preserving mitochondrial integrity and promoting cell survival. The balance between these opposing proteins determines whether a cell will undergo apoptosis, and dysregulation of this balance, particularly Bcl-2 overexpression, is frequently observed in cancers and is associated with resistance to apoptosis and poor prognosis (Alberts, 2022; Assawasuparerk et al., 2016).
In this study, the normal control (NC) group exhibited a higher Bax expression level relative to Bcl-2, reflecting a healthy apoptotic balance in normal breast tissue. Conversely, in the PC group, exposure to the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) led to a relative increase in Bcl-2 expression. DMBA is known to bind to DNA and induce mutations in key regulatory genes, including proto-oncogenes and tumor suppressors such as p53. These alterations can dysregulate apoptotic signaling, increase Bcl-2 expression, and promote uncontrolled cell proliferation and tumor development. Therefore, identifying compounds that restore the apoptotic response by upregulating proapoptotic proteins and downregulating anti-apoptotic ones is a key strategy in cancer therapy.
Natural compounds from Holothuria scabra, including holothurin A and B, have demonstrated diverse anticancer mechanisms in previous studies. These compounds have been shown to exert cytotoxic and proapoptotic effects in various human cancer cell lines, such as gastric and colorectal cancer, lung cancer, and leukemia (Pangestuti and Arifin, 2018; Sajwani, 2019). Our earlier in silico work also indicated that holothurin could function as a tumor suppressor and apoptosis enhancer (Wargasetia et al., 2020). Additionally, Mashjoor et al. demonstrated that sea cucumber extracts from three different species effectively induced apoptosis in various cancer cell lines (Mashjoor and Yousefzadi, 2019). Pranweerapaiboon et al. (2021) reported similar findings, where HSE increased Bax and reduced Bcl-2 expression in human prostate cancer cells (Pranweerapaiboon et al., 2020). Furthermore, our prior research showed that holothurin can suppress the expression of oncogenic pathways, such as the PIK3CA gene, which is involved in cancer cell survival and proliferation (Ratnawati et al., 2024).
In the present study, HSE administered at a low dose (0.33 g/kg BW; T1 group) failed to increase Bax expression and only slightly decreased Bcl-2 expression. Consequently, the Bax/Bcl-2 ratio remained low (0.34), which is associated with apoptosis resistance and a poor therapeutic outlook. In contrast, HSE at moderate (0.66 g/kg BW; T2 group) and high doses (0.99 g/kg BW; T3 group) effectively increased Bax expression and reduced Bcl-2 expression, resulting in a higher Bax/Bcl-2 ratio indicative of enhanced apoptotic activity. These findings suggest that HSE, particularly at doses ≥0.66 g/kg BW, may be capable of tipping the cellular balance toward apoptosis, thereby inhibiting tumor progression.
The Bax/Bcl-2 ratio is widely recognized as a useful indicator of a cell’s apoptotic potential, with a higher ratio reflecting increased sensitivity to proapoptotic stimuli. Dysregulation of this ratio is a hallmark of many cancers and is an important target for therapeutic intervention. By shifting this balance in favor of apoptosis, HSE demonstrates potential as a natural therapeutic candidate in breast cancer management (Cory and Adams, 2002; Kashyap et al., 2021; Youle and Strasser, 2008).
Although the results are encouraging, this study has several limitations. This study revealed a significant increase in the Bax/Bcl-2 ratio; however, the expression of the individual genes was not statistically significant. This outcome could be attributed to biological variability or the intricate regulation of gene expression. Moreover, apoptosis is governed by multiple signaling pathways and regulatory molecules beyond Bax and Bcl-2. Future studies should investigate additional markers of apoptosis, such as caspase activation, mitochondrial membrane potential changes, and other key regulators, to gain deeper insight into the underlying mechanisms involved. It is also essential to explore the pharmacokinetics, bioavailability, metabolism, and potential toxicity of HSE before considering clinical application. Long-term safety and efficacy studies in animal models and eventually in humans will be necessary to facilitate these findings to clinical implementation.
CONCLUSIONS
In conclusion, this study demonstrates that HSE may promote apoptosis in breast cancer tissue by modulating the expression of Bax and Bcl-2, shifting the cellular environment toward programmed cell death. Although further validation is required, particularly with additional molecular endpoints, the findings support the potential of HSE as a promising natural adjuvant therapy. Leveraging Indonesia’s rich marine biodiversity, HSE could serve as a valuable addition to modern oncological strategies, especially for patients seeking alternative or complementary therapies with a traditional basis.
AUTHORS CONTRIBUTION
Conception and Design: Hana Ratnawati and Teresa Liliana Wargasetia. Data Collection: Dhimas P. R. Syahputra, Jasmine Wijaya. Data Analysis and Interpretation: Hana Ratnawati, Ardo Sanjaya. Manuscript Drafting: Hana Ratnawati, Teresa Liliana Wargasetia, Ardo Sanjaya. Supervision: Hana Ratnawati, Teresa Liliana Wargasetia. All authors have read and agreed to the published version of the manuscript.