1. INTRODUCTION
For thousands of years, sophisticated traditional medical systems have been based on plants, leading to the isolation of different chemical compounds and used for various applications (Nasim et al., 2022). Plant-based medications are becoming more popular due to the fact that they are safe, clinically effective, tolerated well by patients, less expensive, and competitive worldwide (Pratt et al., 2019). Aquatic plants, such as bryophytes, pteridophytes, and angiosperms, grow entirely or in part on the water’s surface. They negatively impact aquatic habitats, causing financial losses. Research on aquatic plants has revealed that they are abundant in bioactive compounds and have strong antibacterial effects (Bushmann and Ailstock, 2006). As a result, aquatic plant species may offer various sources for lead compounds that are potent antimicrobials.
Out of 0.4 million plant species worldwide, 47,513 species (11.4% of the total flora) are found in India, which makes up 2.4% of the world’s landmass (Singh and Dash, 2014). India is home to most of the world’s aquatic angiosperms, which are mainly distributed in the Nymphaeaceae, Podostemaceae, Najadaceae, Hydrocharitaceae, Lemnaceae, Alismataceae, Potamogetonaceae, and Ceratophyllaceae families, including 107 aquatic angiosperm species (Arisdason and Lakshminarasimhan, 2016).
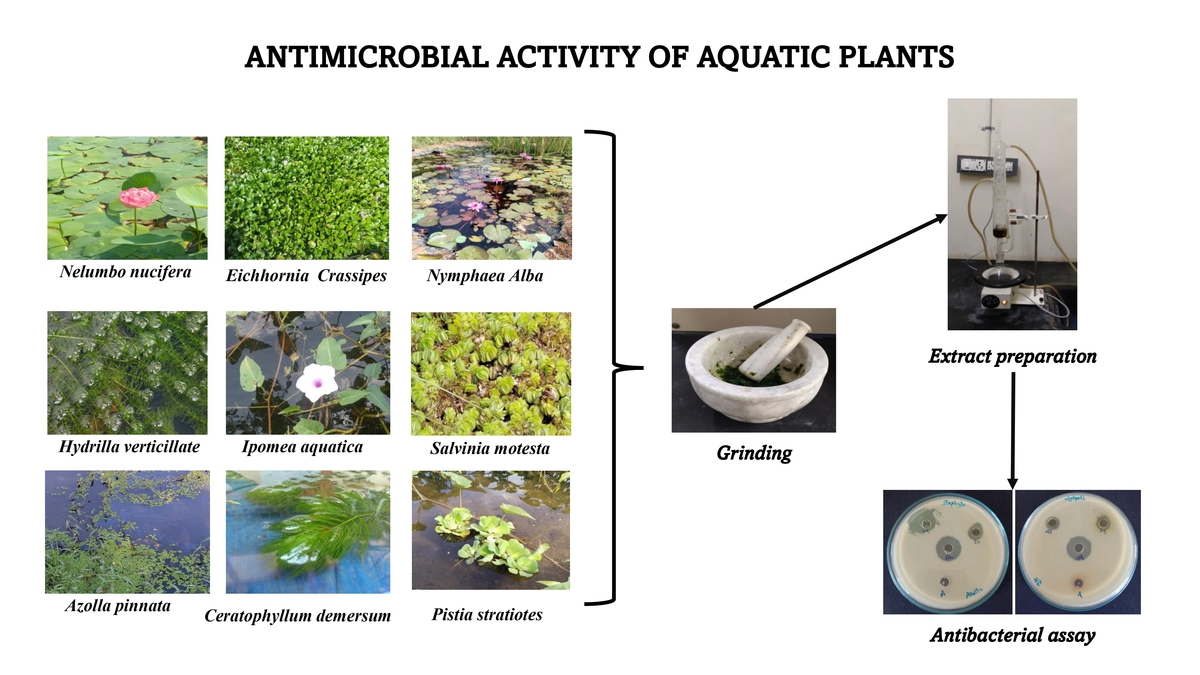
Numerous authors have documented the antibacterial qualities of aquatic plants (Hossain et al., 2018), but to our knowledge, most of the investigations were to evaluate the antibacterial potential of wild plants (Bhaigybati et al., 2020; Verma et al., 2021). Nonetheless, research has indicated that aquatic plants from India possess antimicrobial qualities (Pushkareva et al., 2017). The reports on the ethnomedical survey provided some of the information on aquatic plants used for traditional medical purposes in rural India (Dabur et al., 2007). Understanding the chemical composition of aquatic plants is crucial as it varies considerably based on the species, season, and location (Lata and Dubey, 2010). This study set out to evaluate the antibacterial qualities of different solvent extracts for nine distinct aquatic plants (Figure 1).
2. MATERIALS AND METHODS
2.1. Plant materials
Nine different aquatic plants were used in this study and collected from ponds close to the Department of Animal Science, Manonmaniam Sundaranar University, and the Kandiyaperi Lake, Palaya Pettai in Tirunelveli district (Table 1). The collected plants were cleaned, rinsed in distilled water and shade-dried.
Table 1
Antibiotic sensitivity profile of aquatic pathogens.
2.2. Preparation and preservation of plant extract
The water extraction process was carried out by Rahman et al. (2009). Using the percolation method, 100 g of fresh leaves from each plant were weighed, ground, and immersed in 400 mL of distilled water for 7 days, shaking occasionally. After filtering, the supernatant was lyophilized at low pressure. The dried extracts were diluted in dimethyl sulfoxide (DMSO) to reach a concentration of 50 mg/mL. The dried extract was kept at 4°C for the antibacterial activity test.
Methanol and ethyl acetate (1:10%) together with 100 g of air-dried plant powder were combined. The combination (residue) was macerated overnight in a percolator. After filtering, the extract was boiled in a water bath at 40°C. The concentration of each extract (50 mg/mL) was determined after the dry extract was weighed. For an antibacterial activity test, the resulting extracts were stored at 20°C. (The extraction procedures for two solvents are the same.)
2.3. Test microorganisms
Eight bacterial strains were used in this study. It includes Aeromonas hydrophila, A. caviae, Vibrio cholerae, and Edwardsiella tarda (aquatic pathogen); Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, and Klebsiella pneumonia (human pathogen). All the examined strains were taken from the Department of Microbiology, Manonmaniam Sundaranar University, Tirunelveli. The bacterial cultures were maintained at 4°C on nutrient agar slants and 37°C in nutrient broth, respectively.
2.3.1. Inoculum preparation
Each bacterial strain was subcultured in Mueller Hilton agar (MHA) slants overnight at 37°C. A spectrophotometer was used to harvest the bacterial growth using 5 mL of sterile saline water, correct for absorbance at 580 nm, and diluted to obtain a viable cell count of 1 × 106 Colony Forming Units (CFU)/mL.
2.3.2. Determination of antibacterial activity
To evaluate the antibacterial activity of the aforementioned extracts, the agar well diffusion method was employed with a few modifications by Patra et al. (2009). Five 8.0 mm diameter wells were aseptically created on the assay plates using the wide end of sterile 1 mL tips and the target microbe was then seeded into each well. The crude extracts were reconstituted in DMSO to a final concentration of 50 mg/mL to avoid the test strains negatively impacting the high toxicity of the two solvents. Following that, 100 mL of each extract was loaded into each well. The negative control was DMSO. After 2 hours to allow for full diffusion, the plates were incubated for the entire night at 37°C. The diameter of the negative control was compared to the diameter of the inhibitory zones, which included the well’s (6 mm) diameter. Each test was performed in triplicate and the average of the three repetitions for each extract was determined.
2.3.3. Antimicrobial susceptibility test
The antimicrobial susceptibility assay was performed in MHA plates using the disc diffusion method (Kirby-Bauer disc diffusion susceptibility test protocol). Eight (four human and four aquatic) pathogens were evaluated individually against 16 antibiotics. The National Committee For Clinical Laboratory Standards’ (NCCLS’) disc diffusion method (NCCLS, 2000) was used to determine the results. Following are the tested antibiotics and their respective concentration ranges: ampicillin (10 µg), streptomycin (10 µg), erythromycin (10 µg), penicillin (10 µg), tetracycline (10 µg), doxycycline hydrochloride (10 µg), gentamycin (10 µg), neomycin (10 µg), cloxacillin (10 µg), chloramphenicol (10 µg), oxacillin (10 µg), azithromycin (10 µg), methicillin (10 µg), vancomycin (10 µg), amikacin (10 µg), polymyxin (10 µg), rifampicin (10 µg), ciprofloxacin (10 µg), kanamycin (10 µg), and bacitracin (10 µg). The NCCLS had established the same resistance breakpoints (NCCLS, 2000).
2.3.4. Statistical analysis
The results are shown as three replicates’ mean and standard deviation (SD). SAS (v 6. 12) was used to conduct the statistical analysis. A one-way analysis of variance (ANOVA) at probability level p ≤ 0.05 was used to statistically examine the collected data to determine the level of significance.
3. RESULTS
3.1. Antibacterial activity against tested bacterial pathogens
3.1.1. Azolla pinata
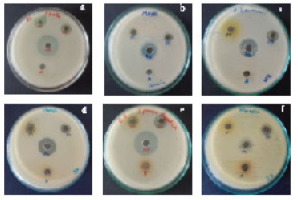
The water extract showed no efficacy against any of the pathogens. The methanol extract exhibited no activity against V. cholera, A. baumannii, or K. pneumonia, but it was most active against S. aureus (20 mm) (Figure 2A), followed by A. caviae (14 mm). The ethyl acetate extract exhibited negligible efficacy against K. pneumonia but had the greatest effectiveness against V. cholera and E. tarda (15 mm). The antibiotic tetracycline showed the greatest antibacterial activity against E. tarda (27 mm) and minimum activity against K. pneumoniae and A. baumannii (16 mm).
3.1.2. Salvinia molesta
The aqueous extraction showed no antibacterial activity against any of the pathogens. The methanol extract was most effective against A. caviae (11 mm) and E. tarda (18 mm). A. hydrophila (8 mm) exhibited the least amount of activity against ethyl acetate extract, while E. tarda (21 mm) exhibited the most. Tetracycline was the antibiotic with the highest action against V. cholera (29 mm) and the lowest against K. pneumoniae (15 mm) (Figure 2B).
3.1.3. Pistia stratiotes
None of the pathogens in this study showed any action in the water extract. V. cholera (8 mm) and A. caviae (10 mm) were the pathogens against which the methanol extract had the greatest action, whereas other pathogens exhibited no activity. Ethyl acetate extract displayed maximum antibacterial efficacy against P. aeruginosa and A. baumannii (12 mm) (Figure 2C) and none against A. hydrophila and S. aureus. Tetracycline was the most effective antibiotic against V. cholera (27 mm) but did not affect A. caviae.
3.1.4. Ipomea aquatica
All the pathogens exhibited no activity in the aqueous extract. Methanol extract had little efficacy against P. aeruginosa and K. pneumonia; however, it was highly active against E. tarda (13 mm) and S. aureus (12 mm). The ethyl acetate extract exhibited the least amount of activity against K. pneumonia and S. aureus (12 mm) and the most against A. baumannii (17 mm). Tetracycline, an antibiotic, exhibited the least amount of efficacy against K. pneumonia (12 mm) and the most against V. cholera, E. tarda, and A. hydrophila (26 mm) (Figure 2D–F).
3.1.5. Nelumbo nucifera
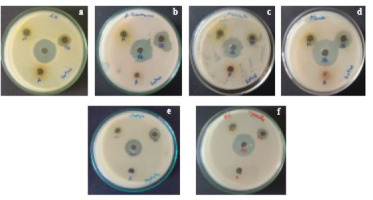
The aqueous extract had no effect against any of the pathogens in Nelumbo nucifera. The methanol extracts showed little effect against A. baumannii and E. tarda, but they were most active against P. aeruginosa (13 mm) and S. aureus (12 mm). Maximum activity was demonstrated by the ethyl acetate extract against P. aeruginosa (16 mm), followed by A. baumannii (15 mm); the least activity was demonstrated against E. tarda (11 mm). Tetracycline had the highest activity against P. aeruginosa (27 mm) and the lowest against A. hydrophila and A. caviae (22 mm) (Table 2 and Figure 3A–D).
Table 2
Antibacterial activity of aquatic plants extracts against aquatic pathogens.
3.1.6. Hydrilla verticillate
The aqueous extraction in this study had no efficacy against any of the pathogens. The methanol extract had no activity against A. hydrophila, A. baumannii, or S. aureus; however, it did exhibit maximum activity against A. caviae and E. tarda (11 mm). Ethyl acetate extract had the least activity against A. hydrophila (9 cm) and the most against P. aeruginosa (15 mm). Tetracycline showed the least activity against K. pneumonia (15 mm) and A. baumannii (16 mm) and the most against A. caviae, V. cholera, and E. tarda (26 mm) (Table 2 and Figure 3E–F).
3.1.7. Eichhornia crassipes
Aqueous extracts showed no efficacy against any of the pathogens in this study. Maximum activity against A. hydrophila (10 mm) and P. aeruginosa (10 mm) was demonstrated by the methanol extract. A high level of activity against P. aeruginosa (12 mm) was demonstrated using ethyl acetate extracts, but no activity against S. aureus was detected. Tetracycline exhibited the least activity against A. hydrophila (23 mm) and the most against A. baumannii (26 mm) (Table 2).
3.1.8. Nymphaea alba
The aqueous extracts of N. alba displayed no activity against all pathogens. The petroleum ether extract had the maximum antibacterial activity against E. tarda (12 mm), whereas the minimum against V. cholera (9 mm) and none was found in A. baumannii and K. pneumonia. The highest activity was shown against V. cholera, E. tarda, and P. aeruginosa (13 mm) with ethyl acetate extract; no activity was detected against A. baumannii and S. aureus. Tetracycline revealed the maximum activity against P. aeruginosa (27 mm), followed by S. aureus (25 mm), and the minimum antibacterial activity against K. pneumonia (20 mm) (Table 2).
3.1.9. Ceratophyllum demersum
C. demersum water extract has shown little efficacy against all pathogens. A. caviae (12 mm) and E. tarda (8 mm) were the two pathogens against which the methanol extracts exhibited the highest activity, whereas the rest of the pathogens exhibited no activity at all. A. caviae (12 mm) and V. cholera (11 mm) were the two organisms against which ethyl acetate extract had the highest antibacterial activity. Tetracycline displayed the maximum activity against V. cholera and E. tarda (26 mm) and the minimum against K. pneumonia (15 mm) (Table 2).
3.2. Antibiotics sensitivity test
The results of standard antibiotic sensitivity tests on aquatic and human pathogens are given in Table 3. All aquatic pathogens from this study showed resistance against erythromycin and methicillin, whereas gentamicin, chloramphenicol, amikacin, ciprofloxacin, and neomycin were found to be sensitive. Among the four aquatic pathogens, three such as A. caviae, V. cholera, and E. tarda were found to be intermediate in kanamycin. Likewise, all human pathogens exhibited resistance against ampicillin, penicillin-G, oxacillin, and methicillin. In this study, gentamicin and amikacin showed sensitivity against all human pathogens whereas polymyxin exhibited intermediate results against all human pathogens.
Table 3
Antibiotic sensitivity profile of human pathogens.
Table 4
Antibacterial activity of aquatic plant extracts against human pathogens.
4. DISCUSSION
Research on novel bioactive compounds for antibacterial properties has increased due to pathogen resistance to antibiotics. Numerous manufacturers of unidentified natural substances in the aquatic ecosystems are thought to have the ability to reduce or control bacterial infections (Aboukhalaf et al., 2020). Some water plants have antibacterial properties that are reported for future applications (Fareed et al., 2008). One advantage of exploring aquatic plants is their quick growth that facilitates the production of large extracts to isolate active chemicals (Wolff et al., 2008). A majority of aquatic plants possess an abundance of bioactive substances that are used in biological processes (Abu-Lafi et al., 2019). The aquatic plants selected for this study are found throughout India. When compared to terrestrial plants, aquatic plants have fewer reports. Therefore, it is important to investigate their applications and conduct comprehensive, broad research to understand their therapeutic properties.
Globally, numerous researchers have assessed the antibacterial properties of diverse aquatic plants (Baral and Vaidya., 2011; Vadlapuli, 2010). E. crassipes water extracts demonstrated strong antibacterial activity against every tested bacterium; these findings were consistent with those from a previous study by Chand et al. (2014). The antibacterial efficacy of water hyacinth methanolic and aqueous extracts against several bacterial strains was revealed by Rufchaei et al. (2021), which exhibited the strongest efficacy among them. These findings contradict the present results because there was no activity in aqueous extracts. The methanolic extracts of E. crassipes showed a significant zone of inhibition (12–17 mm) against the gram-positive bacteria S. aureus (Kiristos et al., 2018). This differed from the finding of our study because there was no antibacterial potential in methanolic extracts of E. crassipes and no recorded activity in methanolic extract against S. aureus. This result is similar to Gutiérrez -Morales et al. (2017) who reported the same results in methanolic extract against S. aureus. Haggag et al. (2017) studied the methanol extract of E. crassipes against P. aeruginosa in which the inhibition zone was less. These results were similar to our findings. Joshi and Kaur (2013) reported the antibacterial activities of aqueous extract in E. crassipes. The results revealed that aqueous extract has weak antibacterial potential but our study indicated no activity in aqueous extracts.
Al-Maliki et al. (2017) reported that N. alba ethanolic extract had high effectiveness against S. aureus. According to our study, N. alba’s methanolic extract was less effective against S. aureus. Daboor and Haroon (2012) recorded the alcoholic extract of N. alba against S. aureus showing that the inhibition zone was very high. The alcoholic extract of N. alba exhibited reduced effectiveness against S. aureus in our study. The effectiveness of N. alba’s aqueous extract against S. aureus, K. pneumonia, and P. aeruginosa was assessed by Yildirim et al. (2013). The findings showed that the aqueous extract exhibited a modest level of antibacterial activity against S. aureus, whereas no activity was found in P. aeruginosa and K. pneumonia compared to our study.
Saraswathi et al. (2019) revealed that water extracts of N. nucifera have moderate activity in S. aureus, but our study found that there was no activity in the water extracts of N. nucifera. Paudel and Panth (2015) reported aqueous and ethyl acetate extract of N. nucifera against S. aureus. The results revealed that aqueous extract has no activity and only moderate activity was found in ethyl acetate. This result was similar to our findings, with more or less the same activity against S. aureus. Kumar and Gitika (2014) studied and compared methanolic and aqueous extracts of N. nucifera, and the results indicated that the methanolic extract was the most effective since it demonstrated the largest inhibitory zone. However, according to our research, ethyl acetate has more potent antibacterial properties than methanol, while the aqueous extract showed no action. Arjun et al.’s (2012) documented methanol extracts showed moderate activity in S. aureus, and these findings agree with our study of methanol extracts against S. aureus. Our investigation found that ethyl acetate had considerable antimicrobial activity, while a similar study by Li and Xu (2008) showed that the methanol extract recorded the maximum antibacterial activity.
According to Pandi Prabha and Rajkumar (2015), H. verticillata ethanolic extract had a positive inhibitory activity for bacteria. In our investigation, methanol and ethyl acetate have shown moderate and strong antibacterial activity, while aqueous extracts showed none. Likewise, Kumari and Kumari (2013) documented the methanolic extracts of H. verticilata against S. aureus and K. pneumonia. The result revealed that the inhibition zone of two pathogens was moderate. But in our study, no activity was found in S. aureus and only moderate activity was present in K. pneumonia. The antibacterial properties of C. demersum stem and leaf methanolic extracts were reported by Omar et al. (2017). The results showed potent antimicrobial activity in pathogens, but our study revealed only moderate antimicrobial activity, and that the methanol extract of C. demersum against S. aureus had no inhibition zone. This result was similar to the study reported by Malathy and Stanley (2015) of methanol extract of C. demersum against S. aureus. Fareed et al. (2008) reported aqueous and methanolic extracts of C. demersum against P. aeruginosa, K. pneumonia, and S. aureus. The results revealed that aqueous extracts had good activity and that methanolic extracts had moderate activity.
Methanolic extracts of I. aquatica have shown modest efficacy against S. aureus, according to our investigation. These findings concurred with those of Manvar and Desai (2013), who found that S. aureus was less active. Methanolic and aqueous extracts of I. aquatica were shown to be effective against S. aureus and P. aeruginosa (Dhanasekaran et al., 2010). The findings demonstrated that the aqueous and methanolic extracts both exhibited good efficacy. However, neither the methanolic nor aqueous extracts of I. aquatica showed any efficacy against P. aeruginosa or S. aureus in our investigation. I. aquatica aqueous extract exhibited moderate efficacy against S. aureus, P. aeruginosa, and K. pneumonia (Bhaigybati et al., 2020; Velmurugan and Suresh, 2020); however, no activity was detected in our investigation.
A. pinnata aqueous and methanolic extracts were shown to be effective against both P. aeruginosa and S. aureus by Farook et al. (2019). The findings showed that both aqueous and methanolic extracts had moderate activity. This result differed from our study because methanolic extract had good activity and aqueous extract showed none. The effectiveness of A. pinnata against P. aeruginosa and S. aureus was previously assessed by Kumar et al. (2017) using its methanol and ethyl acetate extracts. The activity was modest in the methanolic extract, whereas the inhibition zone was significant in the S. aureus ethyl acetate extract and moderate in P. aeruginosa. This finding runs counter to our investigation because the ethyl acetate extract has modest action against two pathogens, whereas the methanolic extract exhibits good activity against P. aeruginosa.
Mandal and Mondal (2011) reported aqueous extracts of S. molesta against V. chlorae and K. pneumonia in which no activity was present. Because of its moderate activity, this result differs from that of the methanolic extract and was comparable to our investigation in aqueous extract. P. stratiotes methanolic extract was shown to be effective against P. aeruginosa, K. pneumonia, and S. aureus by Abraham et al. (2014). The result showed less activity in the methanolic extract of P. stratiotes. This result was different from our study because no activity was found. Likewise, Mukhtar and Tukur, (2019) studied aqueous extract of P. stratiotes against P. aeruginosa, K. pneumonia, and S. aureus. According to the results, the aqueous extract had good efficacy against S. aureus while others showed no activity. However, according to our investigation, P. stratiotes aqueous extract is inactive against any infections. While there was no activity in our investigation, the aqueous extract of P. stratiotes showed modest effectiveness against P. aeruginosa and S. aureus (Rajapaksha et al., 2020). Rahman et al. (2011) reported a methanolic extract of P. stratiotes against S. aureus in which the inhibition zone was less. This result contradicts our study because no activity was found in the aqueous extract. Our study showed that ethyl acetate extract exhibited significant antimicrobial potential. More research is needed to discover the exact components responsible for the antimicrobial action of these extracts. Aqueous extracts exhibited no antimicrobial activity in our study. Future research could examine how these extracts perform against other infections.
4. CONCLUSIONS
The study conducted on the antibacterial properties of aquatic plants in the Tirunelveli district presents significant findings in the field of natural antimicrobials. Extracts made with ethyl acetate and methanol showed significant antibacterial action against a variety of bacterial species, including aquatic and human diseases, according to the study, which concentrated on nine distinct aquatic plants. The most striking outcome of this research is the superior antibacterial potential of the ethyl acetate extracts, followed by the moderate activity exhibited by the methanolic extracts. In contrast, the aqueous extracts showed no antibacterial activity. These findings are particularly relevant in the context of increasing antibiotic resistance, as they suggest that certain aquatic plants could be valuable sources of novel antibacterial compounds. The use of these plant extracts, especially those derived from ethyl acetate, could potentially lead to the development of new drugs for treating infectious diseases caused by bacterial pathogens. Furthermore, this research underscores the importance of exploring local biodiversity— in this case, aquatic plants from specific regions of southern India—for potential medical applications.
DATA AVAILABILITY
This published article contains all the data created or analyzed during this investigation.
AUTHORS CONTRIBUTION
K.K.: Performed the experimental work and developed the overall study design. K.S.: Supervised the research, contributed to experimental planning, and fine-tuned methodology.
R.T.: Assisted in laboratory experiment. A.M.B.: Supported the drafting and organization of the manuscript content. V.A.: Provided critical corrections and refinement of the final article.