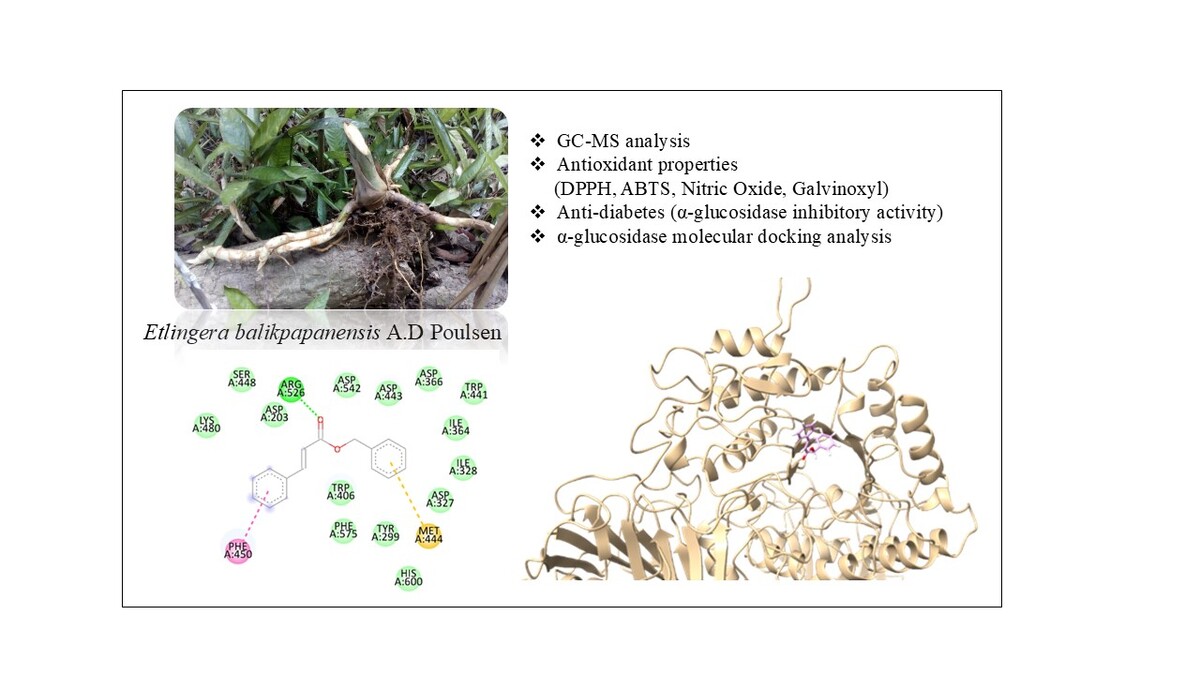
1. INTRODUCTION
Etlingera is a genus of perennial herbs belonging to the ginger family, Zingiberaceae. It comprises over 100 species distributed across continental Asia, Malaysia, Australia, and the western Pacific. These species are traditionally consumed and utilized for medicinal purposes (Chan et al., 2011; Poulsen, 2006, 2012). The leaves, stems, and rhizomes of Etlingera are commonly used as spices or additional ingredients in culinary practices, as well as in traditional therapies to treat various ailments (Ud-Daula et al., 2019). More specialized applications include the use of Etlingera baramensis S. Sakai & Nagam rhizomes in perfumes, Etlingera elatior (Jack) R.M. Sm. and Etlingera pyramidosphaera (K. Schum.) R.M. Sm. fruits in shampoos, and Etlingera coccinea (Blume) S. Sakai & Nagam rhizomes as spices (Ud-Daula et al., 2015). In Borneo, indigenous populations utilize various species, including E. elatior, E. brevilabrum (Valeton) R.M. Sm., E. sessilanthera R.M. Sm., and E. pyramidosphaera, for the treatment of urinary disorders, headaches, abdominal pain, snake bites, and diarrhea (Poulsen, 2016).
These uses align with previous studies that identified the phytochemicals and pharmacological activities of members of this genus, such as E. sayapensis A.D. Poulsen, E. elatior, E. pubescens (B.L.Burtt & R.M.Sm.) R.M.Sm., E. brevilabrum (Pierre ex Gagnep.) R.M.Sm., E. pavieana (K.Schum.) R.M.Sm., and E. fimbriobracteata, ranging from antioxidant, antimicrobial, and anticancer to lipid peroxidase inhibitory activity (Ghasemzadeh et al., 2015; Jambun-Daniel et al., 2018; Tachai & Nuntawong, 2016). According to Mahdavi et al. (2018), various phytochemical components have been identified from this genus, such as phenolic and carboxylic acids. This finding is consistent with the study of Tachai et al. (2014), which revealed the presence of diarylheptanoids in this genus; it is an aromatic plant traditionally used as medicine to treat nausea, fever, and cough (Khor et al., 2017; Mahdavi et al., 2018). Prior research has indicated that essential oils derived from E. pavieana exhibit antibacterial properties against pathogenic gram-positive bacteria, notably Staphylococcus aureus, Listeria monocytogenes, and Bacillus cereus, when applied at concentrations between 0.1 and 0.3% (v/v) (Naksang et al., 2020). Furthermore, essential oils derived from E. fimbriobracteata have exhibited significant antioxidant properties, as evidenced by their activity against 2,2-diphenyl-1-picryl hydrazyl (DPPH) and ABTS radicals. Additionally, these oils have shown antibacterial efficacy against gram-positive bacteria and fungi (Ud-Daula et al., 2016).
Etlingera balikpapanensisis locally known as “Jahe Balikpapan” and is reported as an endemic plant in Balikpapan, East Kalimantan (Poulsen, 2016). It is a rhizomatous herb with leafy shoots that grows up to 3 m (Figure 1). A previous study reported the presence of phytochemical components in E. balikpapanensis leaf extract using qualitative methods and antioxidant activity (Manurung et al., 2019). Although numerous traditional applications and assertions regarding the biological activities of various Etlingera species exist, there is a paucity of empirical studies conducted to substantiate these claims. This study focused on the rhizomes of E. balikpapanensis to assess the phytochemical components of its essential oils and evaluate the pharmacological effects, including antioxidant and enzyme inhibitory activities, along with an in silico analysis. To our knowledge, this study reports the antidiabetic and antioxidant activities of E. balikpapanensis essential oils derived from rhizomes for the first time.
2. MATERIALS AND METHODS
2.1. Plant material
Rhizomes of E. balikpapanensis were collected from Balikpapan Botanical Garden in Balikpapan City, East Kalimantan (Figure 1). The collected rhizomes were washed, shade-dried, and further processed with the steam hydrodistillation process to yield essential oils. Voucher specimens were deposited in the Balikpapan Botanical Garden. The chemicals used were as follows: ABTS (2,2-azino-bis-[3-ethylbenzothiozoline6-sulphonic acid] di-ammonium salt), DPPH, acarbose, aluminium chloride, sodium nitroprusside, and sodium hydroxide, purchased from Tokyo Chemical Industry Co. Ltd. Meanwhile, Quercetin, Baker’s yeast α-glucosidase, sodium carbonate, naphthyl ethylenediamine dihydrochloride, sulphanilic acid, potassium perchlorate, Folin–Ciocalteu reagent, and p-nitrophenyl-α-D-glucopyranoside (p-NPG) were provided by Sigma-Aldrich. Analytical-grade organic solvents, such as methanol and n-hexane, were purchased from Merck.
2.2. Isolation of Etlingera balikpapanensis essential oils
Essential oils from rhizomes of E. balikpapanensis were extracted using the steam hydrodistillation method with slight modification (Saad et al., 2021). Briefly, 5 kg of fresh rhizome parts were steam hydrodistilled with 4 L of water continuously for 4–5 h. The collected essential oils were dehydrated using anhydrous sodium sulphate, weighed, and stored in a sealed vial at 4 °C before bioassay.
2.3. Analysis using Gas Chromatography-Mass Spectrometry (GC-MS)
The essential oils extracted from the rhizomes of E. balikpapanensis were subjected to analysis using gas chromatography (GC) with an Agilent 7890 GC series apparatus. This system was equipped with an HP-5 silica capillary column, characterized by a composition of 5% diphenyl and 95% dimethyl-polysiloxane, measuring 30 m in length, 0.32 mm in internal diameter, and featuring a film thickness of 250 μm. The GC was coupled with a mass spectrometry (MS) 5977B selective detector, with minor modifications as described by Boulamtat et al. (2021). The oven temperature was initially set to 40 °C and subsequently raised at a rate of 2 °C per minute until it reached 230 °C. Detection was performed in full scan mode across a mass-to-charge ratio (m/z) range of 30–1000. The temperatures of the ion source and the quadrupoles were maintained at 230 and 280 °C, respectively. The identification of the components was conducted through a comparative analysis of their mass spectra against the entries in the NIST 2017 mass spectrometry database. The oil components were identified by comparing their retention indices to those of the homologous series of n-alkanes and authentic standards available in our laboratory (C7–C30 Saturated Alkanes Standard, Sigma Aldrich). This data was further corroborated by existing literature.
2.4. Antioxidant properties
2.4.1. Radical scavenging activity against DPPH
The DPPH radical scavenging test was conducted according to a previous study with slight modifications (Khongkarat et al., 2021). A methanol solution of 0.1 mM DPPH was prepared. Briefly, 20 μL of essential oils at varying concentrations were combined with a 0.1 mM DPPH solution in a 96-well microplate, and the experiment was conducted in triplicate. The mixture was incubated for 30 min in the dark. The absorbance of the mixture was determined by measuring the absorption at 520 nm, and ascorbic acid was used as a positive control. The ability of DPPH to scavenge the samples was determined using the following formula:
Where “X0” signifies the absorbance measurement of the blank, and “X1” indicates the absorbance measurement of the sample.
2.4.2. Radical scavenging activity against ABTS
ABTS free radical scavenging activity was assessed using a methodology adapted from a prior study, with minor modifications (Adouni et al., 2022). The radical ABTS•+ was produced by mixing equal volumes of ABTS solution and potassium persulfate (K2S2O8) at a 1:1 ratio. In a 96-well microplate, 20 μL of essential oils at different concentrations was introduced, followed by the addition of the ABTS•+ solution, with each experimental condition being assessed in triplicate. After a 30-minute incubation period, the decrease in absorbance was assessed spectrophotometrically at a wavelength of 750 nm. The percentage of scavenging activity was determined as the percentage inhibition, utilizing the designated Equation 1.
2.4.3. Radical scavenging activity against nitric oxide
Nitric oxide (NO) was generated from sodium nitroprusside in an aqueous solution maintained at physiological pH, subsequently reacting with oxygen to form nitric ions. This reaction was quantitatively assessed using the Griess reaction methodology (Boora et al., 2014). A volume of 20 μL of various concentrations of essential oils was combined with 30 μL of sodium nitroprusside. Following a 1-hour incubation at room temperature, 100 μL of Griess reagent was introduced to the mixture, which was then subjected to a second incubation period of 10 min. Vitamin C served as the standard reference. The resulting pink chromophore was measured spectrophotometrically at a wavelength of 540 nm. The percentage of scavenging activity was calculated as % inhibition using the specified Equation 1.
2.4.4. Radical scavenging activity against Galvinoxyl
Galvinoxyl (GA) radical scavenging activity was assessed by a modification of a previous method (Shi et al., 2001). Briefly, 160 μL of Galvinoxyl free radical was added to 40 μL of extracts with various concentrations. The mixture solutions were then incubated for 120 min, and the absorbance was measured at 428 nm. A methanolic solution of Galvinoxyl was used as a blank, while vitamin C (ascorbic acid) was used as an antioxidant standard.
2.5. α-Glucosidase inhibitory activity
E. balikpapanensis rhizome-derived essential oils and their α-glucosidase inhibitory tests were conducted following methodologies established in prior research (Thant et al., 2021). Briefly, the test sample with various concentrations was mixed with 0.4 U/mL of α-glucosidase, followed by 50 μL of p-NPG solution. After 20 min of incubation at 37 °C, the mixture was stopped by adding 1 M Na2CO3. Enzymatic activity was quantified by measuring the absorbance at 405 nm in a 96-well microplate reader. The α-glucosidase inhibitory activity was expressed as percentage inhibition and calculated using Equation 1. Acarbose was used as a positive control, and the results were expressed as a half-maximal inhibitory concentration value (IC50).
2.6. Molecular docking analysis
Molecular docking studies were performed to investigate the interactions between the secondary metabolites (the major compositions) of the essential oils of E. balikpapanensis rhizomes to the target protein, N-terminal maltase glucoamylase (Nt-MGAM). This protein plays a critical role in carbohydrate digestion by hydrolyzing α-glucosidic linkages in starch, making it a relevant as a target for hyperglycemia in type 2 diabetes mellitus. Therefore, inhibiting Nt-MGAM can help reduce glucose absorption, which supports its selection as a biologically relevant antidiabetic target. The protein crystal structure was retrieved from the Protein Data Bank (PDB ID: 3L4W) (Sim et al., 2010). The GOLD program (Genetic Optimization for Ligand Docking) was utilized to perform molecular docking. The ligand structures were built using Open Babel (O`Boyle et al., 2011). Following the preparation of the protein with GOLD, 10 ligands were subjected to docking simulations, targeting specific coordinates (X: 45.3866, Y: 92.3516, Z: 34.822) within a 16 Å radius (Figure 4). The Gold score function was used to rank the binding poses, with 100 poses generated for each ligand. To establish a reference for binding affinity, the known α-glucosidase inhibitor, acarbose, was also docked under the same conditions. The docking results were subsequently visualized and analysed using Discovery Studio and Chimera to interpret the binding modes and interactions (BIOVIA, 2024; Meng et al., 2023).
2.7. Data analysis
The quantitative data obtained were analysed utilizing descriptive statistics. All measurements were conducted in triplicate. The quantitative data were systematically organized into tables and subsequently exported to SigmaPlot 12.5 for one-way ANOVA analysis, with a significance threshold established at p ≤ 0.05. The resulting quantitative data underwent descriptive analysis.
3. RESULTS AND DISCUSSION
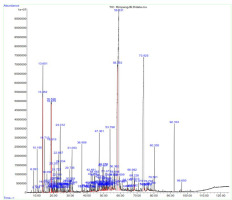
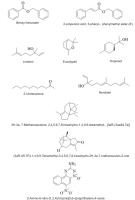
This study investigated the phytochemical composition, antioxidant activity through free radical scavenging (DPPH, ABTS, nitric oxide, and galvinoxyl), and antidiabetic effects by inhibiting α-glucosidase of the essential oils extracted from the rhizomes of E. balikpapanensis, along with their molecular docking analysis. The essential oils, extracted via hydrodistillation, exhibited a pale yellow color. The yield of the essential oils was determined to be 0.04% (v/w based on fresh weight). The GC-MS analysis of the essential oil from E. balikpapanensis rhizomes revealed a total of 62 compounds. Among these, benzyl benzoate (15.24%), 2-propenoic acid, 3-phenyl-, phenylmethyl ester, (E) (14.63%), eucalyptol (3.06%), linalool (8.74%), terpineol (2.98%), and Z, E-nerolidol (2.14%) were identified as the major components. Additionally, the potential antioxidant properties and antidiabetic effects of α-glucosidase inhibition were investigated. Furthermore, the major components of the essential oils were selected for further analysis through molecular docking.
3.1. Essential oil analysis
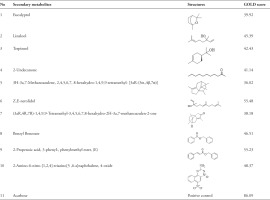
The yield of essential oils of E. balikpapanensis rhizomes was determined to be 0.04% (v/w based on fresh weight), which is slightly lower than the yields reported for other rhizomes of Etlingera pavieana, which range from 0.28 to 0.70% (v/w based on dry weight) (Tachai et al., 2014; Vairappan et al., 2012). Mahdavi et al. (2017) reported that the yield percentage of essential oils from the rhizomes of Etlingera sayapensis was 1.51% (w/w%). The yield of essential oils extracted from the rhizomes of E. balikpapanensis was found to be greater than the yields previously documented for the rhizomes of E. fimbriobracteata and E. brevilabrum, which were reported to be 0.03 and 0.02% (v/w on a dry weight basis), respectively (Mahdavi et al., 2012; Ud-Daula et al., 2016). Studies have demonstrated that the chemical composition and yield of essential oils obtained from Etlingera species can be influenced by abiotic factors such as seasonality, water availability, light exposure, temperature, plant developmental stage, and nutritional status, as well as by genetic factors and practices during and after harvest (Naksang et al., 2020; Wong et al., 2010). These factors may be associated with the lower yield of essential oils observed in this study. The GC-MS analysis was performed to assess the qualitative profile of different chemical compositions found in the essential oils extracted from E. balikpapanensis rhizomes. The identification of secondary metabolites in this research relied solely on the similarity index derived from the NIST 2017. Table 1 shows the obtained results for a total of 62 volatile compounds identified in the rhizome part, each with a similarity index of 90% or higher. Out of the compounds found in E. balikpapanensis rhizome essential oils, 10 major components (>1% abundant), as well as their retention time, peak areas, similarity index, and relative retention indices (RRI) from experiments and literature, are listed in Table 2, presenting mass spectrometry library, with the incorporation of alkane series for determining retention indices (Figure 2). These 10 major components (Figure 3) include benzyl benzoate, 2-propenoic acid, 3-phenyl-, phenylmethyl ester-(E), linalool, eucalyptol, terpineol, Z,E-nerolidol, 2-undecanone, and 3H-3a,7-methanoazulene, 2,4,5,6,7, and 8-hexahydro-1,4,9,9-tetramethyl-(3aR-[3α.,4β,7α]). However, the essential oils of E. balikpapanensis were dominated by benzyl benzoate (15.24%), while 2-propenoic acid, 3-phenyl-, phenylmethyl ester, (E) represented only 14.63% of the oils. This result aligns with the findings of the study conducted by Ud-Daula and Basher (2019), who identified nonterpenoid compounds as the predominant phytochemical category present in essential oils derived from the rhizomes of E. pavieana, Etlingera brevilabrum, Etlingera cevuga (Seem.) R.M.Sm., Etlingera linguiforme (Roxb.) R.M.Sm., Etlingera littoralis (J. Koenig) Giseke and Etlingera coccinea (Blume) S. Sakai & Nagam. Previous research has indicated that the rhizomes of various Etlingera species, including E. pavieana, E. fimbriobracteata, E. elatior, E. littoralis, and E. sphaerocephala (Griff.) R.M.Sm., contain compounds such as terpineol, nerolidol, linalool, eucalyptol, and 2-undecanone (Mahdavi et al., 2013; Naksang et al., 2020; Ud-Daula et al., 2016; Wong et al., 2010). Essential oils extracted from the rhizomes of E. balikpapanensis, as analyzed in this study, display a composition that closely resembles that of the previously mentioned species. Mahdavi et al. (2013) conducted an analysis of hydrodistilled essential oil from the dried rhizomes of E. brevilabrum sourced from Malaysia, identifying eucalyptol (27.6%), terpineol (0.3%), and 2-undecanone (2.0%) among the compounds present. Furthermore, the essential oil of E. balikpapanensis was found to contain small quantities of terpenes, such as monoterpene hydrocarbons, sesquiterpene hydrocarbons, and terpenoids, including oxygenated monoterpenes and oxygenated sesquiterpenes, which are a class of organic compounds. This observation aligns with the research conducted by Yahya et al. (2010) and Susanti et al. (2013), who documented elevated levels of nonterpenes in essential oils extracted from the rhizomes of various Etlingera species, including Etlingera cevuga, E. elatior, and E. fulgens. A comparative analysis of essential oil components from various Etlingera species revealed that their chemical compositions are influenced by factors such as the specific plant species, the plant parts utilized, the sources of the materials, and the methods employed in their preparation (Abdelwahab et al. 2010). The investigation into the chemotaxonomy of various Etlingera species revealed a significant similarity in the predominant compound groups present in their essential oils. The majority of these oils are characterized by a high concentration of oxygenated monoterpenes and monoterpene hydrocarbons. Conversely, certain species exhibit sesquiterpene hydrocarbons, phenylpropanoid derivatives, and phenolic compounds as the primary compound groups. Despite this, considerable variation was noted in the overall compositions of the essential oils. Nevertheless, β-pinene, terpineol, and 2-undecanone are identified as common principal compounds across the volatile oils of most species within this genus. To date, no additional studies have been published regarding the chemical compositions of essential oils derived from the rhizome of E. balikpapanensis.
Table 1
Phytochemicals of the essential oils from Etlingera balikpapanensis rhizomes.
Table 2
Major secondary metabolites identified from the essential oils Etlingera balikpapanensis rhizomes.
[i] RRIx: Relative retention indices experimentally calculated against n-alkanes. RRIy: Relative retention indices from literatures: a (Siani et al., 2004); b (Ramos et al., 2000); c (Gopanraj et al., 2005); d (Kahriman et al., 2010); e (Sabulal et al., 2006); f (Hong-Liang et al., 2007); g (Sylla et al., 2009); h (Yayli et al., 2005); i (Utsunomia et al., 2005); j (Zeng et al., 2007); k (Feizbakhsh & Naeemy, 2011); l (Andriamaharavo, 2014)
3.2. Antioxidant properties
To investigate the inhibitory effects of essential oils from the rhizome of E. balikpapanensis on antioxidant activities, we performed an in vitro study to scavenge free radicals such as DPPH, ABTS, nitric oxide, and galvinoxyl. The antioxidant properties of essential oils derived from the rhizomes of E. balikpapanensis are summarized in Table 3. Unfortunately, the essential oils exhibited no inhibitory activity against DPPH free radicals compared to ascorbic acid, which served as a positive control with an IC50 value of 5.21 ± 0.16 mg/mL. This observation aligns with the findings of Abdelwahab et al. (2010), who documented a limited DPPH free radical scavenging activity in the essential oils derived from the entire E. elatior plant. Additionally, Riahi et al. (2013) noted that the essential oils extracted from E. fimbriobracteata exhibited inferior radical scavenging activities compared to established positive controls. Several studies have indicated that the antioxidant efficacy of essential oils is contingent upon the presence of phenolic compounds (El-Massry et al., 2002). Moreover, the antioxidant capacity of specific medicinal plants was assessed using the ABTS•+ free radical method. The IC50 values for ABTS•+ free radical scavenging by essential oils (3.39 ± 0.04 mg/mL) were almost similar to those of ascorbic acid used as a positive control (4.59 ± 0.03 mg/mL). The results of this study closely align with those of the research conducted by Sabli et al. (2012), which reported that the rhizomes of Etlingera velutina exhibited higher antioxidant activity. The ABTS•+ discoloration assay is a widely utilized method for assessing the antioxidant activity of essential oils. Although both DPPH and ABTS assays are based on similar principles, the antioxidant activity of essential oils measured by the ABTS assay is typically lower than that observed in the DPPH assay. This discrepancy may be attributed to steric factors and a double bond in the side chain, which stabilizes the DPPH radical through resonance (Jing et al., 2012). This result complies with those of the DPPH test, indicating that essential oils from the rhizomes of E. balikpapanensis are more effective in trapping cationic free radicals ABTS•+. To the best of our knowledge, there have not been reports on the scavenging activity of essential oils from the rhizomes of E. balikpapanensis on ABTS•+ radicals. Moreover, the antioxidant efficacy of essential oils derived from the rhizomes of E. balikpapanensis was assessed with nitric oxide free radicals. Nitric oxide (NO) is a significant contributor to inflammatory processes. Excessive NO production results in tissue damage as well as acute and chronic inflammation. Chronic inflammation induced by nitric oxide free radicals has been linked to a range of diseases, including juvenile diabetes, arthritis, and ulcerative colitis (Venkatachalam & Muthukrishnan, 2012). Consequently, agents that inhibit nitric oxide free radicals, such as essential oils, may prove advantageous in managing inflammatory responses. In this study, essential oils demonstrated significant antioxidant activity against nitric oxide free radicals, as indicated by the IC50 values presented in Table 3. Ascorbic acid, utilized as a positive control for the nitric oxide radical scavenging assay, exhibited scavenging activity with an IC50 value of 4.38 ± 0.02 mg/mL. The mechanism by which essential oils exert their effects involves inhibition of nitric oxide free radicals through the suppression of nitrite formation during the radical generation process, by competing with oxygen (Habu & Ibeh, 2015). The findings suggest that the essential oil extracted from the rhizomes of E. balikpapanensis exhibits a significant capacity to scavenge various free radicals across different systems. This property implies that the oil may have potential applications in preventing pathological damage associated with free radicals, particularly in mitigating the degradation of biomolecules and DNA caused by lipid peroxyl and NO radicals, which are implicated in conditions such as arteriosclerosis, carcinogenesis, and inflammation (Venkatachalam & Muthukrishnan, 2012). The antioxidant efficiency of the essential oil extracted from the rhizomes of E. balikpapanensis was also evaluated against galvinoxyl free radicals. In the galvinoxyl assay, the essential oil showed antioxidant activity that was four times weaker than the positive standard (ascorbic acid). Galvinoxyl radical was widely employed to evaluate the ability of an antioxidant to donate a hydrogen atom to the oxygen radical (Tai et al., 2014). Furthermore, the intricate chemical composition of essential oils, which encompasses various components characterized by distinct functional groups, polarity, synergistic or antagonistic response, and chemical properties, complicates the elucidation of the diverse forms of antioxidant activity (El-Ghorab et al., 2010; Yang et al., 2010). This study is the first to provide evidence and recognize the scavenging activities of essential oil extracted from the rhizomes of E. balikpapanensis, sourced from East Kalimantan, against various free radicals, including DPPH, ABTS, nitric oxide, and galvinoxyl.
Table 3
Biological activities of the essential oils from Etlingera balikpapanensis rhizomes.
3.3. α-Glucocidase inhibitory activity
In light of the correlation between excessive free radicals and complications associated with diabetes, an α-glucosidase inhibitory test was conducted on essential oils derived from the rhizomes of E. balikpapanensis. α-Glucosidase is a crucial enzyme involved in the hydrolysis of oligosaccharides. Inhibiting this enzyme represents a significant strategy for regulating blood glucose levels. Evaluating the antidiabetic properties of essential oils is vital for identifying alternative substances with low toxicity and minimal side effects for the treatment of diabetes mellitus. The α-glucosidase inhibitor efficacy of essential oils extracted from E. balikpapanensis rhizomes was evaluated by comparing their resulting IC50 values, as indicated in Table 3. High IC50 values indicated low inhibitory activity. The essential oils exhibited inhibitory activity against α-glucosidase with an IC50 value of 6.16 ± 0.06, slightly higher than that of the positive standard, acarbose (IC50 5.36 ± 0.09 mg/mL). Additionally, Tran et al. (2024) reported that essential oils of rhizomes from two species in the Zingiberaceae family—Wurfbainia schmidtii (K.Schum.) Škorničk. & A.D.Poulsen and Zingiber atroporphyreus Škorničk. & Q.B. Nguyên showed antidiabetic activities against α-glucosidase and α-amylase. Tran et al. (2024) also reported that the essential oils from Alpinia nelumboides showed antidiabetic activities by inhibiting α-glucosidase. Furthermore, the antidiabetic properties of essential oils may be attributed to their primary constituents. Previous research indicates that essential oils, which are abundant in nonterpenoid compounds such as phenols and various aromatic molecules, play significant biological roles in the management of diabetes and possess antioxidant properties. These compounds have the potential to improve insulin sensitivity, lower blood glucose levels, and mitigate oxidative stress, a critical contributor to diabetes and its associated complications (Bungau et al., 2023). Terpenes found in the essential oils made from E. balikpapanensis rhizomes, such as β-pinene, also have the potential to inhibit critical enzymes associated with type 2 diabetes, particularly α-glucosidase. It was reported that administering terpenes to diabetic patients exerts α-glucosidase inhibitory activity, and essential oils of Alpinia vietnamica rhizomes exhibit high antioxidant activity (Tra et al., 2023). Moreover, essential oils consist of intricate combinations of various molecules, raising the question of whether their biological effects stem from the synergistic interactions among all constituents or are predominantly influenced by the principal molecules identified in higher concentrations through gas chromatography. A review of the existing literature revealed a lack of reports regarding the in vitro α-glucosidase inhibitory activity of E. balikpapanensis. Conversely, within the Etlingera genus, there are documented instances of species assessed for their antidiabetic properties, including the flowers of E. elatior (Nor et al., 2020).
3.4. Molecular docking analysis
To evaluate the antidiabetic potential of essential oil constituents from E. balikpapanensis rhizomes, molecular docking simulations were also conducted against the N-terminal maltase glucoamylase using the GOLD software. The primary compounds (Table 2) identified in the essential oils of E. balikpapanensis, as determined by GC-MS, were subsequently analyzed through molecular docking simulations, with acarbose serving as the reference inhibitor. The GOLD score reflects the predicted binding affinity, where a higher score indicates stronger and potentially more biologically relevant binding. This study aims to not only compare binding affinities but also explore the binding poses and key molecular interactions with the enzyme’s active site, thereby supporting future inhibitor design.
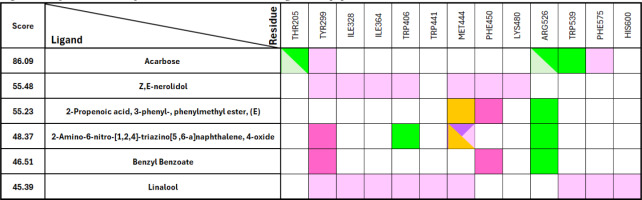
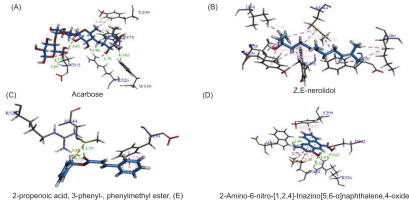
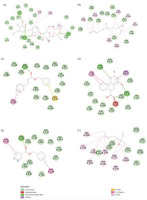
The docking analysis revealed that acarbose, the standard ligand, exhibited the highest GOLD score of 86.09, indicating a strong binding affinity. This finding is further supported by various types of interactions, as detailed in Tables 4 and 5. As illustrated in Figures 4 and 5, acarbose forms six hydrogen bonds with key residues, including Thr205, Arg526, and Trp539, with bond distances ranging from 1.79 to 3.03 Å. Additionally, acarbose interacts through van der Waals forces with Thr205 and Arg526, and establishes hydrophobic contacts with Tyr299 (4.40 Å) and Phe575 (5.04 Å) (Figure 6A). These diverse interactions underscore the ability of acarbose to engage with critical residues, reinforcing its stability and enhancing its potential efficacy. Among the essential oil compounds, Z,E-Nerolidol, which attained a score of 55.48, can form 12 hydrophobic interactions with several residues, including Tyr299, Ile328, Trp406, Met444, Phe450, and Lys480, with bond distances ranging from 4.14 to 5.21 Å (Figure 6B). The ligand 2-propenoic acid, 3-phenyl-, phenylmethyl ester, (E), with a score of 55.23, interacted with Arg526 through two hydrogen bonds, each distancing 1.72 and 2.75Å. Other interactions, such as Pi-Sulphur interactions with Met444 (5.18Å) as well as Pi-Pi stacking with Phe450 (3.38Å), were also observed (Figure 6C). Additionally, 2-Amino-6-nitro-[1,2,4]-triazino[5,6-a] naphthalene, 4-oxide, which scored 48.37, formed four hydrogen bonds with Trp406 and Arg526 with distance ranging from 1.66 to 2.99Å, and demonstrated Pi-Pi stacking interactions with Tyr299 (3.77 & 5.73Å). This ligand also interacted with Met444, forming Pi-Sigma (2.79Å), hydrophobic (5.23Å), and Pi-Sulphur contacts (3.49Å), indicating a complex binding profile (Figure 6D). Benzyl benzoate, scoring 46.51, participated in a single hydrogen bond with Arg526 and exhibited Pi-Pi stacking interactions with Tyr299 and Phe450. Linalool, on the other hand, relied exclusively on hydrophobic interactions with residues such as Tyr299, Ile328, Ile364, Trp406, Trp441, Met444, Trp539, Phe575, and His600. Although none of the essential oil compounds surpassed acarbose in GOLD score, the docking results revealed diverse binding poses and interaction profiles that suggest their potential as complementary or alternative inhibitors of N-terminal maltase glucoamylase (NtMGAM). Several compounds demonstrated interactions with key active site residues, particularly hydrogen bonds with Arg526 and hydrophobic interactions with Tyr299, which were also prominently involved in acarbose binding, indicating shared molecular recognition features and structural compatibility, as shown in Table 5. These observations highlight the ability of certain natural compounds to engage the active site through hydrophobic contacts, hydrogen bonding, and π-interactions, suggesting possible modulatory effects on enzyme activity. These findings contribute to a structure-based understanding of how essential oil constituents from E. balikpapanensis bind to NtMGAM as antidiabetic agents, paving the way for further studies involving optimization, analog development, or combination strategies with existing inhibitors.
Figure 5
The 2D view of the top 5 scored ligands of the essential oil from Etlingera balikpapanensis rhizomes. (A) Acarbose, (B) Z,E-Nerolidol, (C) 2-Propenoic acid, 3-phenyl-, phenylmethyl ester, (E), (D) 2-Amino-6-nitro-[1,2,4]-triazino[5,6-a]naphthalene, 4-oxide, (E) Benzyl Benzoate, (F) Linalool.
4. CONCLUSION
This study presents an inaugural investigation into the chemical compositions, antidiabetic properties, antioxidant activities, and in silico analysis of the rhizome essential oils derived from E. balikpapanensis. The assessment of antioxidant and α-glucosidase inhibitory activities revealed significant efficacy of the essential oils, a finding corroborated by molecular docking analyses. Consequently, it is recommended that the potential application of E. balikpapanensis rhizome essential oils in food products and nutraceuticals targeting diabetes be explored further. Given that the essential oils of this species are utilized in the treatment of various ailments, additional research into the efficacy of the rhizome essential oils is warranted.