1. INTRODUCTION TO ENDOPHYTES AND THEIR METABOLIC POTENTIAL
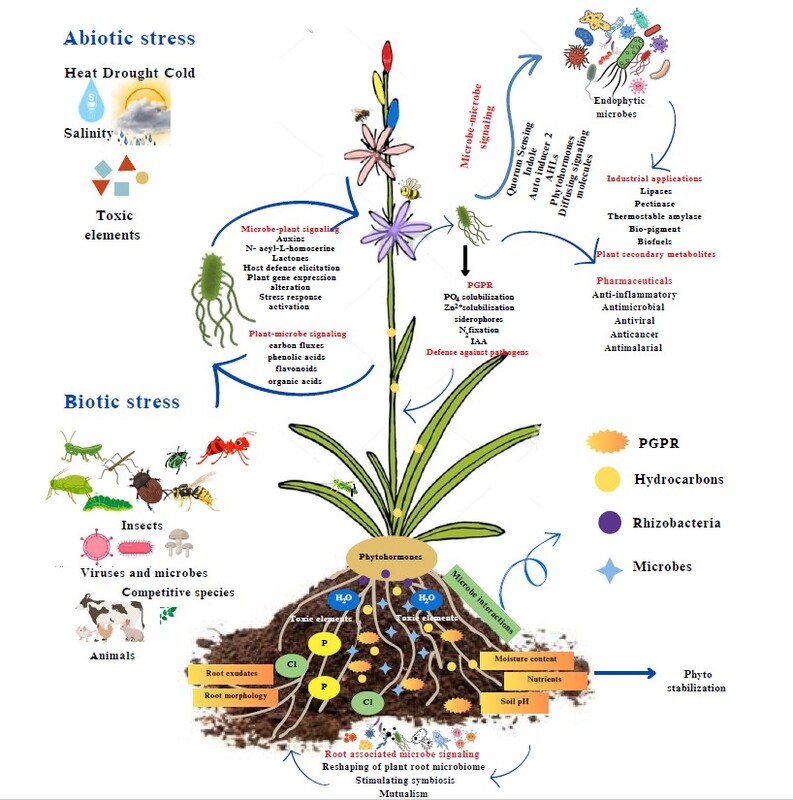
Around 300,000 plant species exist in the world; they are home to unexplored, mysterious microbial endophytes. Endophytes are microbes that live inside plants, aiding in plant growth and development. The study of plant-microbe interactions has recently garnered significant interest in the scientific community (Vishwakarma et al., 2020; Muhammad et al., 2024). Endophytes are now known to boost plant health by facilitating nutrient uptake, improving plant resilience to changing climate, targeting pathogens and pests, and enhancing plant defenses (Afzal et al., 2019; A. K. Singh et al., 2022).
Endophytes help plants acquire nutrients, including iron, nitrogen, and phosphorous, from the environment or by generating phytohormones such as indole acetic acid (IAA), gibberellic acids, ethylene, and auxins for plant growth and development (Santoyo et al., 2016; Firdous et al., 2019; Mehta et al., 2019; Singh and Kumar, 2023). Nitrogen fixation is a well-known function in actinorhizal and rhizobial plant symbionts such as Frankia, Azospirillum, and Rhizobia species (Lindström and Mousavi, 2020). Endophytes are also known to produce siderophores like catecholate and hydroxamate, which aid in iron acquisition (Aznar et al., 2015). Several researchers have demonstrated the vital role of endophytic microbes in increasing plant resilience to abiotic stresses, including drought, toxic elements, and agrochemicals (Kumari et al., 2023; Zakariyah et al., 2024). Endophytes are also known to help defend against plant pathogens (Donald et al., 2005; Zhang et al., 2012; Singh et al., 2020).
The intricate plant-microbiome interactions are known to regulate plant secondary metabolic pathways that lead to the synthesis of flavonoids, alkaloids, and terpenoids in plants (Singh and Kumar, 2023; Toppo et al., 2023; Zakariyah et al., 2024). Recent studies have uncovered a fascinating aspect of endophytes: their capability to produce secondary metabolites independent of their host (Mishra et al., 2021). This discovery holds remarkable promise compared to traditional methods of extracting phytochemicals, which often involve the overexploitation of plants. These metabolites have immense value for use in pharmaceuticals, cosmetics, and agriculture. Moreover, endophytes can be used specifically to promote the production of certain metabolites in plants, such as increasing the yield of bioactive alkaloids in medicinal plants or enhancing flavonoid content for improved antioxidant properties. Employing endophytes to increase the yield of these compounds—either through direct synthesis by the endophytes or by enhancing the host plant’s natural biosynthetic pathways—presents an innovative and eco-friendly solution to meet the growing demand for high-value phytochemicals. Though some reviews have presented the role of endophytes in the regulation of secondary metabolite production, this review sheds light on the advancements in omics technologies that have catalyzed recent research in this field (Li et al., 2023; Tsipinana et al., 2023a). Moreover, this review bridges conventional bioprocessing approaches and modern strategies that could be exploited to enhance beneficial plant metabolites (Venugopalan and Srivastava, 2015b; Ganeshan et al., 2021b; Verma et al., 2023). The current review delves into the latest advancements and outlines future directions in harnessing endophytes to produce plant secondary metabolites, focusing on their exciting potential for sustainable biotechnology applications.
2. ROLE OF ENDOPHYTES IN PRODUCTION OF PLANT SECONDARY METABOLITES
2.1. Alkaloids
Alkaloids, a type of nitrogen-containing compound, are classified as amines and amides, indole derivatives, pyridines, and quinazolines, as well as quinoline and isoquinoline. Several alkaloids have shown remarkable insecticidal, antimicrobial, cytotoxic, or anticancer activities (Zhang et al., 2012). The endophyte Acremonium zeae from maize produces pyrrocidine alkaloids with significant antifungal activity against Fusarium verticillioides and Aspergillus flavus, which can be used to prevent contamination by fumonisin and aflatoxin in preharvest maize (Donald et al., 2005; Mishra et al., 2021). Endophytic strains from Catharanthus roseus cultivar Dhawal enhance alkaloid production in the plant. Strains identified as Choanephora infundibulifera and Curvularia sp. increased serpentine content by several hundred-fold, while others like Aspergillus japonicus and Pseudomonas sp. boosted ajmalicine content by 20 to 100%. It was proposed that these endophytes could increase the synthesis of serpentine and ajmalicine by modifying the expression of key biosynthetic genes in the terpenoid indole alkaloid pathway (Singh et al., 2020). Several other researchers have documented the production of novel alkaloids by endophytes (Table 1).
Table 1
Alkaloids from endophytes
| Secondary metabolite | Endophyte | Plant | Bioactivity | Reference |
|---|---|---|---|---|
| 10 -methoxy-60 -epi-oxysporidinone, ()-4,60 -anhydrooxysporidinone, sambutoxin | Fusarium concentricum | Anoectochilus roxburghii | Anticancer (HT29 and PC3 cell line) | (Zhang et al., 2024) |
| 4a-methyl-dodecahydro-1H-pyrrolo[3,4-b] quinoline-6-one | Aspergillus niger | Dodonaea viscosa | Antibacterial- against B. coagulans, S. aureus, K. pneumoniae, E. coli and P. aeruginosa | (Ramesh et al., 2022) |
| Alkaloids | Choanephora infundibulifera, Curvularia sp ., Pseudomonas sp . & Aspergillus japonicus | Catharanthus roseus | Increased serpentine and ajmalicine content | (Singh et al., 2020) |
| Pantothenic | Neurospora sp ., Entrophospora infrequens. | Nothapodytes foetida | Antitumor activity | (Singh et al., 2021) |
| Fumigaclavine C& pseurotin A | Aspergillus sp. | Bauhinia guianensis | Antibacterial action | (Pinheiro et al., 2013) |
| Homoharringtonine | Alternaria tenuissima | Cephalotaxus sp. | Anticancer human chronic myeloid leukemia and antitumor activity | (Hu et al., 2016) |
| 1,11-dideacetyl-pyripyropene A, pyripyropene A, pyripyropene E, tryptoquivaline J, chaetominine, fumitremorgin C, 1-acetyl-β-carboline, and nicotinic acid | Aspergillus fumigatus | Rhizophora mucronata | Immunosuppressive and cytotoxic | (Zou et al., 2022) |
| Pyrrocidines A and B | Acremonium zeae | Maize | Antifungal activity-Aspergillus flavus & Fusarium verticillioides | (Donald et al., 2005) |
| Vinblastine | Curvularia verruculosa | Catharanthus roseus | Cytotoxic effect on HeLa cell line | (Parthasarathy et al., 2020) |
| Vincristine | Nigrospora zimmermanii | Catharanthus roseus | Anti-cancer | (Birat et al., 2022) |
2.2. Flavonoids
Flavonoids are a broad category of phenolic secondary metabolites primarily found in plants but also produced by several microorganisms. Aspergillus oryzae and Aspergillus nidulans, isolated from the branches of Ginkgo biloba L., and Deinococcus sp. obtained from the Ginkgo rhizosphere are capable of producing flavonoid compounds (Qiu et al., 2010; Zhou et al., 2023). Derivatives of flavonoids extracted from Juniperus cedre’s Nodulisporium sp. exhibited antibacterial properties and are being further investigated for the synthesis of antibiotics (Dai et al., 2006). The flavonoid-producing endophytic fungus from the branches of Loranthus tanakae showed strong antioxidant and antibacterial activities (Zheng et al., 2022). Quercetin, a flavonoid extracted from endophytic bacteria Serratia, Neisseria, Acinetobacter, and Yersinia sp., exhibits anticancer and antimicrobial activity (Ramadhan et al., 2018) (Table 2).
Table 2
Flavonoids from endophytes.
| Secondary metabolite | Endophyte | Plant | Bioactivity | References |
|---|---|---|---|---|
| 2',4'-dihydroxy-6'-methoxy-3',5'-dimethylchalcone | Ceriporia lacerata | C. operculatus | Anti-cancer effect | (Wang et al., 2013) |
| 3-methoxyflavone, daidzein, nobiletin, formononetin and scopoletin | Chaetomium & Xylariaceae sp. | Conyza blinii H. Lév | Antioxidant property | (Tang et al., 2020) |
| Cajanol | Hypocrea lixii | Cajanus cajan [L.] Millsp. | Cytotoxicity activity towards A549 cells | (Zhao et al., 2013) |
| Flavonoid derivatives | Nodulisporium sp. | Juniperus cedrus | Antimicrobial | (Dai et al., 2006) |
| Quercetin | Serratia, Neisseria, Acinetobacter, Yersinia spcs. | Cosmos caudatus kunth. | Anticancer and antimicrobial activity. | (Ramadhan et al., 2018) |
2.3. Quinone
Quinone is a yellow crystalline compound with a chlorine-like odor, slightly soluble in various solvents. It acts as an oxidizing agent and can be reduced to hydroquinone (Devi and Mehendale, 2014). Endophytes, fungi, and bacteria are known producers of several quinones with potential agricultural and medicinal applications. For example, endophytes synthesize compounds like altersolanol A, which shows antimicrobial effects against S. epidermidis, S. aureus, and Enterococcus faecalis by acting as an electron acceptor in bacterial membranes (Aly et al., 2008). Perylenequinones (PQ) are known for their inherent antiviral, antimicrobial, anticancer, and photodynamic properties (Y. Chen et al., 2022) (Table 3).
Table 3
Quinones from Endophytes.
| Secondary metabolite | Endophyte | Plant | Bioactivity | References |
|---|---|---|---|---|
| 1,3,8-trihydroxy-6-methylanthraquinone | Polyporales sp. | Rheum emodi | Cytotoxic effect (A549, THP-1(Leukemia), NCI-H322 (lung) and Colo-205(colon) | (Dar et al., 2017) |
| 6-O-demethyl-4-dehydroxyaltersolanol A | Nigrospora sp. | Aconitum carmichaeli | Antiviral | (Zhang et al., 2016) |
| Anthraquinones | Coniothyrium sp. | Salsola oppostifolia | Anticancer, antioxidant, laxative | (Sun et al., 2013) |
| Perylenequinones | Shiraia sp. | - | Antimicrobial, anticancer | (Y. Chen et al., 2022) |
| pyrroloquinoline quinone | Burkholderia seminalis | - | Antifungal (protect bananas from Fusarium wilt) | (Hung et al., 2024) |
2.4. Terpenoids
Terpenoids have a variety of biological properties, such as antimicrobial, hyperglycemic, cytotoxic, and antitumor effects. They also promote plant root growth, induce quinone reductase enzyme activity, and studies in humans have shown that they provide latent hepatic protection (Zhao et al., 2020a, 2020b; Y. Chen et al., 2021). Recent studies show that endophytic microorganisms can produce terpenoid compounds. Approximately 200 terpenoids, including monoterpenoids, sesquiterpenoids, triterpenoids, and meroterpenoids, have been identified from plant endophytic fungi such as Aspergillus, Phomopsis, Penicillium, Trichoderma, Bipolaris, and Paraconiothyrium. Cadinene sesquiterpenes derived from the endophyte Phomopsis cassiae of the plant Cassia spectabilis showed high antifungal action against Cladosporium sphaerospermum and C. cladosporioides (Silva et al., 2006) (Table 4).
Table 4
Terpenes from Endophytes.
| Secondary metabolite | Endophyte | Plant | Bioactivity | References |
|---|---|---|---|---|
| Azadirachtin | Eupenicillium parvum | Azadirachta indicaA. Juss. | Insecticides | (Kusari et al., 2014) |
| Cadinene sesquiterpenes | Phomopsis cassia | Cassia spectabilis | Antifungal against Cladosporium sphaerospermum | (Silva et al., 2006) |
| Terpenoids | Aspergillus, Trichoderma, Penicillium, Bipolaris, Phomopsis, and Paraconiothyrium | - | Anti-bacterial, anti-pathogenic, anti-fungal, cytotoxic, anti-tumor and hyperglycemic | (Zhao et al., 2020b) |
| Xiamycin | Streptomyces sp. | Bruguiera gymnorrhiza | Anti-HIV activity | (Ding et al., 2010) |
| xylarenic acid, xylarenones A and B | Xylaria sp. | Torreya jackii Chun. | Antitumor and antibacterial activity | (Hu et al., 2008) |
2.5. Peptides
Peptides play vital roles in numerous biological processes, including hormone regulation, immune response, and antimicrobial defense (Forbes J, Krishnamurthy K., 2025). In an interesting study, the endophytic strain Penicillium sp. isolated from Acrostichum aureum was shown to produce antibacterial peptides cyclo (Pro-Tyr) and cyclo (Pro-Thr) (Cui et al., 2008). Significant antibacterial activity was demonstrated by Streptomyces sp. isolated from shoots of Atropa belladonna, which produced congeners of two known hexapeptides that included piperazic acid (PA) (Bekiesch et al., 2021) (Table 5).
Table 5
Peptides from endophyte.
| Secondary metabolite | Endophyte | Plant | Bioactivity | References |
|---|---|---|---|---|
| collutellin A | Colletotrichum dematium | Pteromischum sp. | Antifungal activity against Botrytis cinerea and Sclerotinia sclerotiorum | (Ren et al., 2008) |
| Cyclo (Pro-Tyr) and Cyclo (Pro-Thr) | Penicillium sp. | Acrostichum aureurm | Antibacterial activity | (Cui et al., 2008) |
| (3 S,4 R)-dihydroxy-(6 S)-undecyl-α-pyranone and cyclo-(l-Phe-l-Leu1-l-Leu2-l-Leu3-l-Ile) | - | mangrove Avicennia marina | Inhibitory effect on human cancer cell line Bel-7402 | (Li et al., 2004) |
| Peoriaerin II | Paenibacillus peoriae | Millettia pachycarpa Benth | Antimicrobial | (Ngashangva et al., 2021) |
| talaromins A and B | Talaromyces wortmannii | Aloe vera | Antimicrobial | (Bara et al., 2013) |
2.6. Phenolics
Phenolics are compounds that have an aromatic hydrocarbon structure, often a benzene ring, linked to one or more hydroxyl groups (-OH) (Lin et al., 2016). Phenolic compounds isolated from endophytes demonstrate significant antioxidant and antifungal activities. For example, pestalachloride A29 and B30 from Pestalotiopsis adusta effectively combat pathogens Gibberella zeae, Fusarium culmorum, and Verticillium aiboatrum (Li et al., 2008). Pharmacologically active phenolic compounds, p-hydroxyphenyl acetic acid and ferulic acid, obtained from the endophytic fungus Aspergillus sp., demonstrated remarkable antioxidative effects. Ferulic acid showed both antioxidant and modest antifungal action against A. niger (Abonyi et al., 2018) (Table 6)
Table 6
Phenolics from endophytes.
| Secondary metabolite | Endophyte | Plant | Bioactivity | References |
|---|---|---|---|---|
| Polyphenols | Bacillus mycoides and Bacillus cereus | Urtica dioica | Antioxidant, anti-cancerous, and anti-inflammatory | (Marchut-Mikołajczyk et al., 2023) |
| 2,4-Di-tert-butylphenol | N. sphaerica | Euphorbia hirta L | Antioxidant | (Gautam et al., 2022) |
| cosmochlorins D and E | Phomopsis sp. | Ficus ampelas | cytotoxicity (HL60 cells) | (Shiono et al., 2017) |
| Ferulic acid | Aspergillus sp. | Moringa oleifera | Antioxidant antifungal | (Abonyi et al., 2018) |
| Pyrogallol | Annulohypoxylon stygium | Asparagopsis taxiformis | Antimicrobial (E. coli, MRSA) | (Medina et al., 2019) |
| Tyrosol, p-hydroxyphenylacetamide | Coriolopsis rigida | Cochlospermum regium | Antioxidant allelopathic | (Dantas et al., 2023) |
2.7. Steroids
Steroids are isoprenoid lipids present in fungi, plants, and animals and have a variety of biological functions (Dembitsky, 2023). The coculture of the endophytic bacterium Bacillus wiedmannii Com1 and endophytic fungi Pleosporales sp. was reported to produce the ergosterol derivative 23R-hydroxy-(20Z,24R)-ergosta-4,6,8(14),20(22)-tetraen-3-one and its related compound (22E,24R)-ergosta-4,6,8(14),22-tetraen-3-one. Among these, 23R-hydroxy-(20Z,24R)-ergosta-4,6,8(14),20(22)-tetraen-3-one exhibited antibacterial efficacy against Staphylococcus aureus (Abdalla and Matasyoh, 2014; Wang et al., 2019) (Table 7)
Table 7
Steroids from endophytes.
| Secondary metabolite | Endophyte | Plant | Bioactivity | References |
|---|---|---|---|---|
| (22 E)-3α,6α,9α-ergosta-7,22-diene-3,6,9-triol | Periconia pseudobyssoides | Toona sureni | Heme polymerization inhibition activity | (Azhari et al., 2023) |
| 2β, 6β-dihydroxy-5α-methoxyergosta-7, 22-diene, 2β, 9α-dihydroxy-5α-methoxyergosta-7, 22-diene | Fusarium solani | Chloranthus multistachys | Antagonistic activity | (Shen et al., 2016) |
| Ergosterol derivatives | Co-cultures of Pleosporales sp.and Bacillus wiedmannii | - | Inhibits microbial activity | (Wang et al., 2019) |
| lactone aspergilolide, verruculogen, tryprostatin B, helvolic acid, 13-oxofumitremorgin B, demethoxy fumitremorgin C, fumitremorgin C, aszonalenin, 12, 13-dihydroxy-fumitremorgin C, terezine D | Aspergillus sp. | Paeonia ostii | Cytotoxic activity | (Zhang et al., 2019) |
| Pestalachloride A and B | Pestalotiopsis adusta | - | Antifungal activity – Gibberella zeae and Fusarium culmorum | (Li et al., 2008) |
Their capability to influence plant metabolism through signaling pathways highlights their importance in agricultural sustainability. Overall, endophytes are a promising resource for bioactive metabolites that not only enhance plant health but can also be explored for their commercial applications in diverse fields. Several patents disclosing innovative approaches for the production of secondary metabolites and biotransformation processes involving endophytic fungi have been granted, underlining the growing applications. The most frequently identified referenced genera in these patents include Fusarium, Trichoderma, Aspergillus, Phomopsis, and Penicillium. The secondary metabolites obtained from these endophytes demonstrate a diverse range of applications, such as anticancer, antimicrobial, antifungal, neuroprotective, and antioxidant agents (Torres-Mendoza et al., 2020). Moreover, some of these metabolites are precursors for producing pharmaceutically vital compounds (Kumar et al., 2019). (Table 8).
Table 8
Patented commercial product derived from fungal metabolite.
| Patent number | Product/ Metabolite | Endophyte source | Plant source | Biological application | References |
|---|---|---|---|---|---|
| US 2004O248265A1 | Podophyllotoxin | Phialocephala fortinii strains | Podophyllum species | Anticancer activity | (Porter and Eyberger, 2004) |
| US6329193B1 | Taxol | Cladosporium macrocarpon | Taxus spp. | Anticancer activity | (Strobel and Daisy, 2004) |
| US20040185031A1 | Naphthalene | Muscodor vitigenus | Paullinia paullinioides | Insect repellent | (Strobel and Daisy, 2004) |
| US20040206697A1 | Organic volatile antibiotics | Muscodor albus | Cinnamon tree | Treatment of harmful microbes from human & animal waste. | (Strobel and Ezra, 2006) |
| CN1850765A | Quinone compounds | Halorosellinia sp | Mangrove | Antitumor activity. | (She et al., 2006) |
| US7192939B2 | 1,5,7 trisubstituted 1,3-dihydroisobenzofuran & derivatives | Pestalotiopsis microspore | Terminalia morobensis | Antioxidant and antimycotic | (She et al., 2006) |
| CN101412971A | 5α, 8α-ergosterol peroxide-6, 22-diene-3β-ol (16), ergosterol-8(9), 22-diene-3β, 5α, 6β, 7α-tetraol (17), and succinic acid (18). | Fusarium sp. | Paris polyphylla var. yunnanensis | Antimicrobial activity | (Zhou et al., n.d.) |
| CN102633616A | Preparation of anthraquinone dimer alterporriol P (51) | Alternaria sp. | Sarcophyton sp. | Antineoplastic activity | (Wang et al., 2012) |
3. HOST PLANT-FREE ENDOPHYTE CULTIVATION
3.1. Isolation of endophytes
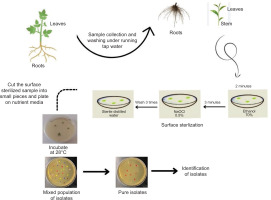
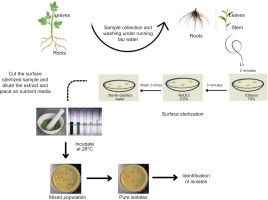
One of the main challenges in isolating endophytes is contamination by surface microbiota. Endophyte diversity must remain unaffected while appropriate sterilization of surfaces is ensured to isolate endophytes. Due to varying requirements for growth conditions, one endophyte may flourish while others may be suppressed, leading to misinterpretation (Dos Reis et al., 2022). The surface sterilization procedure and cultivation on rich microbiological medium at 28°C are the first steps in the isolation of endophytes. Recent investigations have successfully cultivated endophytes using this approach (Eevers et al., 2015a; R. Singh et al., 2022).
Endophyte isolation procedures need to be tailored for the specific plant and the endophyte of interest. In a study, bacterial endophytes isolated from rhizomes of G. perpensa were obtained by sectioning the rhizomes into small pieces, followed by surface sterilization and plating on nutrient agar media (Mahlangu et al., 2024). Another approach involved macerating surface-sterilized root pieces with quartz sand, followed by dilution of the extract before plating on Tryptic Soy Agar (TSA) and TSA with added NaCl. This method proved effective for isolating a broad spectrum of microbial communities, including halophilic bacteria (Mesa et al., 2015). The efficacy of endophyte isolation is highly dependent on the choice of nutrient medium. Many studies investigating the optimization of isolation protocols have found that varying media types significantly impact both the diversity and regrowth capacity of endophytes (Eevers et al., 2015a). These findings highlight the importance of selecting appropriate isolation conditions to capture the full spectrum of endophytic diversity (Figures 1 and 2).
3.2. Common metabolic pathways
Endophytes are key players in the intricate metabolic networks that shape their symbiotic relationships with their host plants, contributing to both plant health and the production of bioactive compounds. These remarkable fungi are capable of synthesizing plant-associated metabolites and their analogues, expanding the potential for novel natural products (Mesa et al., 2015). As the world faces the urgent crisis of plant conservation, especially with endangered species at risk of overharvesting and biodiversity loss, endophytes offer a sustainable alternative. By harnessing their capability to produce the same valuable compounds found in rare plants, we can mitigate the need to exploit these vulnerable species. The revelation that endophytic fungi can generate metabolites akin to those of their host plants marked a revolutionary turning point in natural product research. A striking example of this breakthrough is the finding that Taxol, a potent anti-cancer compound, can be synthesized by an endophyte of Taxus brevifolia (Zhang et al., 2012). This pivotal discovery not only opened new doors for drug development but also highlighted the untapped potential of endophytes as a source of valuable bioactive substances.
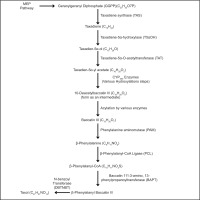
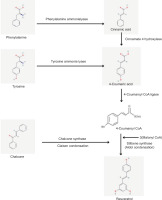
Baccatin III: 3-amino, 3-phenylpropanoyl Transferase (bapt) and taxadiene Synthase (txs) genes were found in the endophyte genome Taxomyces andreanae isolated from Taxus by PCR analysis (Figure 3). The genes bapt and txs encode enzymes that catalyze the first and last steps of the taxol biosynthesis process in plants. The scientists identified both genes in the endophyte genome and found 96% and 97% similarity, respectively, with the corresponding genes in Taxus sp. The plant origin of the txs gene was further confirmed by the presence of an N-terminal plastid targeting sequence, thereby corroborating the horizontal gene transfer (HGT) hypothesis (Mishra et al., 2021). Endophytic fungi use the methylerythritol 4-phosphate (MEP) and mevalonate (MVA) pathways to produce terpenoids from isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) (El-Sayed et al., 2020). These terpenoids enhance plant defense mechanisms and have therapeutic applications, such as producing anticancer substances like Taxol (Sze et al., 2008; Galindo-Solís and Fernández, 2022).
Research into the biosynthesis of perylenequinones by the endophytic fungus Shiraia sp. Slf14(w) has revealed that adding L-arginine can significantly enhance their production by upregulating critical metabolic pathways (Y. Chen et al., 2022). A recent study showed that Priestia megaterium PH3 produces the highest yield, i.e., 947 μg/L of resveratrol for a natural isolate, in just two days (Figure 4). The primary rate-limiting enzyme for resveratrol synthesis was cinnamate 4-hydroxylase (C4H), which is part of the phenylpropanoid pathway. These results provide valuable information for future metabolic engineering projects and demonstrate Priestia megaterium PH3’s potential for industrial resveratrol synthesis (Zhang et al., 2023).
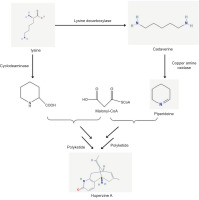
Swainsonine (SW) is the primary poisonous component of locoweed plants and is produced by specific endophytic fungi such as Alternaria, Slafractonia leguminicola, Undifilum oxytropis, and Metarhizium anisopliae. It causes locoism, a disease of sheep, cattle, and horses resulting from locoweed consumption. SW has also been shown to have antitumor and anticancer effects. It causes locoism, characterized by a disorder of the nervous system (Ren et al., 2017). Although exact pathways can vary among fungi, the production of SW in endophytes begins with the conversion of lysine into several chemicals, such as saccharopine and pipecolic acid (Figure 5). Based on genome sequencing and functional investigation, researchers postulate the initial stages of SW production in A. oxytropis and propose important enzymes that might control this process (Ren et al., 2017).
Figure 5
Swansonine biosynthesis pathway in endophytic fungi (Ren et al., 2017). Enzymes involved in the pathway: SDH: saccharopine, FAP2: Saccharopine oxidase, PIPOX: - pipecolate oxidase, P5CR: pyrroline-5-carboxylate reductase, PKS: polyketide synthase, P450: cytochrome P450.
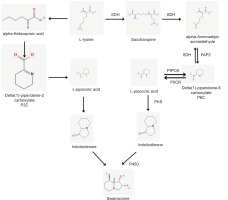
Huperzine A (HupA) is an acetylcholinesterase (AChE) inhibitor obtained from the club moss Huperzia serrata. It is an approved drug in China for Alzheimer’s disease and is used as a nutraceutical in other parts of the world. Over 60 putative biosynthetic gene clusters and more than 33 type I polyketides were identified in the genomic study of Colletotrichum gloeosporioides, an endophyte from Huperzia serrata. The researchers proposed a novel biosynthetic pathway for HupA involving a hybrid polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) mega-enzyme, with pipecolic acid as a precursor (Xia et al., 2022) (Figure 6).
The most common IAA synthesis pathway observed in endophytes is the indole-3-pyruvate (IPyA) pathway (Tian et al., 2015). The plant hormone strengthens the plant immune response against a broad range of pathogens (Bastías et al., 2018). Various characteristics of endophytic microorganisms have made them popular biocontrol agents for protecting crops from phytopathogens (Ahmed et al., 2020). The fungal endophyte Streptomyces sp., which produces 1-aminocyclopropane-1-carboxylate deaminase (ACCD), was found to confer salt resistance in Oryza sativa (Jaemsaeng et al., 2018; Burragoni and Jeon, 2021).
Thus, endophytes may decrease the need for resource-intensive cultivation of medicinal plants; however, altering endophytes outside of their native host setting carries a number of environmental concerns. Introducing endophytes into non-native habitats has the potential to disturb existing microbial communities, alter nutrient cycling, or cause unexpected ecological impacts, such as the introduction of novel genes or metabolites that could influence soil health and native plant-microbe interactions. Endophytic populations’ adaptation is heavily controlled by local climatic conditions and host specificity; hence, their introduction into new environments may result in unforeseen consequences, including possible pathogenicity or diminished efficacy in their new hosts. Furthermore, artificial manipulation or large-scale application of endophytes may disrupt the balance between beneficial and harmful microorganisms, underscoring the necessity for extensive ecological risk evaluations before widespread use (Nair and Padmavathy, 2014; Farrer et al., 2025).
4. OMICS APPROACHES TO STUDY PLANT MICROBIOME
Metagenomic and multi-omics techniques have transformed the discovery of novel endophytes and their metabolic pathways by enabling culture-free, high-throughput analysis of entire microbial populations within plant tissues. Metagenomics, employing next-generation sequencing along with shotgun or metabarcoding approaches, allows identification and functional annotation of unculturable endophytes and the genes involved in bioactive metabolite synthesis, such as antibiotics and plant growth-promoting compounds. Combining metagenomics with transcriptomics, proteomics, and metabolomics provides a comprehensive view of endophyte biodiversity, gene expression, protein function, and metabolite profiles, uncovering the complex dynamics of plant-endophyte interactions. This integrative approach accelerates the discovery of novel metabolic pathways for applications in agriculture, medicine, and environmental sustainability (Nwachukwu and Babalola, 2022; Adeleke et al., 2023; Serepa-Dlamini et al., 2024; Balan et al., 2025).
Recent studies have highlighted the intricate relationships between plant secondary metabolites (PSMs) and plant microbiomes, emphasizing their roles in defense, stress response, and organismal interactions (Pang et al., 2021). Plants actively shape their microbiomes through root exudates, including flavonoids, strigolactones, coumarins, and triterpenes (L. Chen et al., 2021). Omics technologies—genomics, transcriptomics, proteomics, and metabolomics—have revolutionized our understanding of plant-microbe interactions, providing insights into mechanisms such as nitrogen fixation and systemic resistance induction (Malook et al., 2023; Jain et al., 2024). These approaches are critical for developing sustainable agricultural practices and optimizing plant-microbe alliances. Among these, metabolomics has emerged as a powerful tool for evaluating plant species properties and addressing agricultural challenges (Karabulut et al., 2021). Future research should focus on integrating multi-omics data, enhancing technology resolution, and exploring plant-microbe dynamics across diverse conditions to ensure sustainable food production and environmental stewardship (Karabulut et al., 2021; Jain et al., 2024).
Metabolomics, the high-throughput analysis of small metabolites, offers direct insights into an organism’s phenotype and has emerged as a powerful tool in plant-endophyte interaction research (Mishra et al., 2022). This approach has revealed how endophytes modulate plant metabolism, enhancing nutrient availability and stress tolerance (Mishra et al., 2022). Complementing transcriptomics and proteomics, metabolomics provides additional information on regulatory processes and the end products of plant-microbe interactions (Feussner and Polle, 2015). It has also uncovered novel compounds involved in systemic acquired resistance and the formation of barriers against pathogens (Feussner and Polle, 2015). When combined with transcriptomics, metabolomics deepens our understanding of plant-endophyte recognition, colonization, and protective mechanisms (X. Chen et al., 2022). In ecological studies, metabolomics has shed new light on plant resistance to herbivores (Macel et al., 2010). As a functional genomics approach, it has successfully linked phenotypes to genotypes in model plants such as Arabidopsis thaliana (Macel et al., 2010; Karabulut et al., 2021).
5. APPLICATION OF FERMENTATION TECHNOLOGY TO PRODUCE METABOLITES FROM ENDOPHYTIC MICROORGANISMS
The primary challenge in translating beneficial plant-endophyte interactions to industrial applications lies in scaling up endophyte cultures for large-scale production (Ganeshan et al., 2021a). Genomic instability in axenic cultures limits the consistent formation of high-value secondary metabolites, confining production mostly to laboratory settings and hindering commercial scalability (Venugopalan and Srivastava, 2015a). To address these challenges, strategies such as bioprocess optimization, mixed fermentation, co-cultivation, and the use of epigenetic modifiers are being explored to enhance production stability and yield. Moreover, further research into host-endophyte interactions and metabolic regulation is essential to fully harness the commercial potential of endophyte-derived phytochemicals in industrial contexts (Sharma et al., 2021; Toppo et al., 2024).
Research indicates that only about 0.001 to 1% of the total endophytes residing within plant tissues are cultivable under laboratory conditions (Eevers et al., 2015b). Optimizing culture media is therefore crucial for enhancing the growth and metabolite production of isolated endophytes. Numerous studies have demonstrated that the choice of nutrient media significantly affects both the number and diversity of cultivable endophytes. Notably, supplementation of culture media with plant extracts has been shown to substantially increase the diversity of viable endophytes isolated. This effect has been observed in both minimal and rich media, suggesting that crushed plant tissue provides essential components absent in synthetic media but necessary for the growth of certain endophytes (Abdalla and Matasyoh, 2014; Gerna et al., 2022). For example, the production of vinblastine and vincristine by Taxomyces radicus was optimized across nine different liquid media, with high vincristine yields obtained in modified M2 medium and high vinblastine production in potato dextrose broth (Palem et al., 2015; Fikri et al., 2018).
Researchers successfully isolated a wide variety of endophytic bacteria, including more than 50% of the predominant strains, from Dendrobium roots using modified cultivation techniques such as the separate sterilization of phosphate and agar (PS) and the use of gellan gum (GG) as a gelling agent (Nishioka and Tamaki, 2022). Further investigation into the effects of the PS and GG methods on colony formation showed that all tested recalcitrant isolates were recovered on all media types (Nishioka and Tamaki, 2022). However, endophyte growth was significantly enhanced on PS or GG plates compared to the traditional method of autoclaving all components together (Abdalla and Matasyoh, 2014; Nishioka and Tamaki, 2022). In another study, the production of antimicrobial metabolites by endophytic Aspergillus sp. isolated from the root of Calotropis procera was optimized by altering various culture parameters, including carbon and nitrogen sources, temperature, pH, and incubation time (Verma et al., 2017). Media supplemented with 3 g/L yeast extract resulted in the highest yield of antimicrobial metabolites (Verma et al., 2017).
Microbial lipopeptides are produced by endophytic bacteria but the key challenge in production is low yield and higher cost of production. Industrial production can be made economically viable by development of hyperproducing strains and optimizing upstream and downstream processing, use of cheap raw materials and the optimization of medium-culture conditions (Beltran-Gracia et al., 2017). Xylanases, which are essential for industrial uses, including food, textiles, animal feed, and biofuels, were produced by optimizing solid-state fermentation using Aspergillus niger. The study determined the ideal substrate, media composition, initial pH, and mineral solutions using inexpensive agro-industrial waste (sorghum stover, soyabean meal, wheat bran, and corn cob) in tray bioreactors. It also evaluated for the impact of temperature, moisture content, and particle size on the production of xylanase (Khanahmadi et al., 2018). Lipases are versatile enzymes utilized in a variety of applications, and they can be manufactured using agro-industrial waste via solid-state fermentation. In a study, optimization of lipase production by endophytic Aspergillus ibericus was carried out using solid state fermentation of agro-industrial waste such as olive oil pomace and wheat bran (Oliveira et al., 2018). The key factors assessed were the ratio of carbon to nitrogen (C/N), the amount of lipids and phenols, the composition of sugar, the addition of nitrogen, and the use of a packed-bed bioreactor to enhance the conditions for lipase extraction (Oliveira et al., 2017).
A novel plastic single-use bioreactor with five kg of solid substrate was used in a study to scale up the fermentation process for producing 6-pentyl-α-pyrone (6PP), lytic enzymes, and conidia by Trichoderma asperellum (Rayhane et al., 2019). The single-use bioreactor demonstrated the potential of solid-state fermentation for industrial uses in biopesticide production. The specially formulated media consisted of jatropha cake, olive pomace, vine shoots, and olive oil as substrates at 25°C for seven days. By applying humid air during fermentation, the bioreactor produced optimal yields of 6PP, lipases, amylases, and fungal conidia (Rayhane et al., 2019; Perwez and Al Asheh, 2024).
In a study, optimization of the fermentation condition and medium composition for Fusarium sp. led to a 10-fold increase in N-methylsansalvamide (SA) yield. The ideal parameters were 13 days of fermentation, pH of 6.5, 24°C, 24-hour seed age, inoculum size 5.0% (v/v), 50% (v/v) loading volume, and concentrations of 22.5 g/L sucrose, 0.024 g/L yeast extract, 16.5 g/L tryptone, and 20.0 g/L sea salt. The study shows that SA yield may be effectively increased, and the path to large-scale N-methylsansalvamide (SA) production for new medication advancement can be paved by logically adjusting fermentation conditions and medium composition (Shao et al., 2024).
Thus, recent advances in strain improvement for enhancing secondary metabolite production include amplification or disruption of key gene clusters, inactivation of competing pathways, and manipulation of regulatory genes using tools such as CRISPR/Cas9, ZFNs, and TALENs. These genetic engineering techniques are complemented by modern approaches like genomics, transcriptomics, proteomics, metabolic engineering, and whole genome shuffling, enabling more precise and efficient metabolite pathway optimization. Such innovations provide a robust framework for future developments in synthetic biology and metabolite engineering (Verpoorte and Memelink, 2002; Mipeshwaree Devi et al., 2023; Tsipinana et al., 2023; Verma et al., 2023).
Thus, advancement in synthetic biology, including potential synthesis of complete genome, has prompted serious ethical and safety issues, particularly regarding environmental impacts, moral bounds, ethical access to benefits, and biosecurity hazards. While its applications in environmental remediation are often seen as beneficial in terms of pollution reduction, worries remain concerning unanticipated ecological repercussions and the potential generation of deadly infections. Regulatory frameworks and continuing ethical discourse are important to address these difficulties and enable the responsible development and deployment of synthetic biology technology (https://hudsonlabautomation.com/exploring-the-ethics-and-risks-of-synthetic-biology/, n.d.; Ou and Guo, 2023).
6. CHALLENGES AND LIMITATIONS IN HOST PLANT FREE METABOLITE PRODUCTION
In recent years, the focus on secondary metabolite production from endophytes has shifted towards optimizing culture conditions and exploring innovative techniques such as metabolic engineering, cocultures, and precursor feeding to enhance production. However, challenges persist in translating these advances into practical industrial applications. The limited availability of plant-derived endophytic metabolites, such as taxol, is a major bottleneck due to environmental and geographical factors that affect their quality and quantity. Furthermore, mimicking the complex conditions required for host-free metabolite production remains difficult, leading to inferior yields and quality when compared to natural sources. These obstacles complicate the production of essential secondary metabolites at a scale that meets clinical and commercial demands, highlighting the need for more efficient methods.
The integration of ‘omics’ technologies, including metabolomics and genomics, has provided deeper insights into the genetic and biochemical pathways of endophytes, offering new opportunities to discover and optimize bioactive compounds. Exploring host-free cultivation systems holds promise, but these systems can only partially replicate the natural conditions that influence metabolite synthesis. An alternative approach is using endophytes to stimulate plants, enhancing their natural production of secondary metabolites, which could result in a more sustainable and effective strategy. The combination of omics-based insights, improved fermentation techniques, and plant-endophyte interactions presents a promising path for the large-scale, commercial production of bioactive compounds with applications in pharmaceuticals, agriculture, and beyond. Continued research and technological advancements are key to overcoming existing challenges and unlocking the full potential of endophyte-derived metabolites.
Author Contributions
Research concept and design, Collection and/or assembly of data, Data analysis and interpretation, Writing the article, Critical revision of the article, Final approval of the article: S.S.C., Collection and/or assembly of data, Data analysis and interpretation, Writing the article: V.R.B.