INTRODUCTION
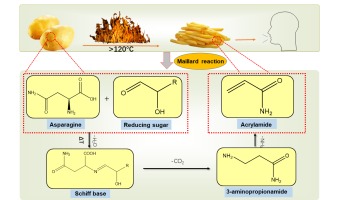
Food safety is a public health issue of global concern and is related to people's life and health. Chemical contaminants in food are an essential factor affecting food safety. A variety of chemical contaminants such as acrylamide (AA), polycyclic aromatic hydrocarbons (PAHs), heterocyclic aromatic amines (HAAs), 3-monochloropropane-1,2-diol esters (3-MCPDE) and N-nitroso compounds (NOCs) are produced during the thermal processing of food (Li et al., 2021). Among them, AA is mainly found in baked goods such as potato chips, bread, cakes, and cookies and is a compound produced by the Maillard reaction during high temperatures (Andačić et al., 2020). During this reaction, free asparagine in the food is condensed with reducing sugars to produce Schiff base, which can be further reacted to produce the intermediate product 3-APA and finally of AA (Figure 1) (Schouten, Tappi, & Romani, 2020).
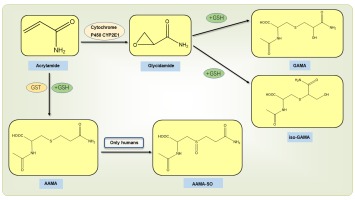
When people consume cakes, cookies, and bread, the AA in them is absorbed into the body by the small intestine's villi. AA is metabolized in the body by two pathways: (1) Under the action of cytochrome P450CYP2E1; AA is metabolized to glycidamide (GA), which is subsequently bound to glutathione and degraded to N-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-L-cysteine (GAMA) and the tautomeric N-acetyl-S-(1-carbamoyl-2-hydroxyethyl)-cysteine (iso-GAMA). (2) Under the action of glutathione-S-transferase (GST), AA binds to glutathione and degrades to produce N-acetyl-S-(2-carbamoylethyl)-cysteine (AAMA), which in humans AAMA is converted to sulfonylated AAMA (AAMA-SO) (Figure 2) (Chen et al., 2020).
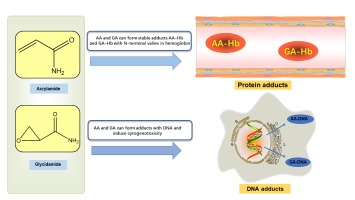
AA and GA can be covalently bound to the N-terminal valine of hemoglobin (Hb) to form stable adducts AA-Hb and GA-Hb (Figure 3), which can remain in the blood for a longer time, which is the reason for the accumulation of AA in the body (Ou, Ou, Huang, Ho, & Ou, 2020). Since 2002, when the Swedish National Food Agency (SNFA) first published the chemical contaminant AA in baked and fried foods, the study of AA toxicity to human health has attracted significant interest from researchers. Existing studies have shown that AA has neurotoxicity, carcinogenicity, reproductive toxicity, genotoxicity, and hepatosplenic toxicity in humans. This paper reviews the latest research progress on the effects of AA on human health in the past three years, describes the formation and metabolic processes of AA in vivo, explores the toxic effects and molecular mechanisms of AA, and clarifies the exposure of AA in humans by risk assessment, and uses detection methods and mitigation measures to detect and reduce AA.
DETECTION METHODS OF ACRYLAMIDE CONTENT IN FOOD
It is crucial to detect AA in foods because of its potential toxic effects on humans. AA, a small molecule compound, is more difficult to detect in complex food matrices. Carbohydrate-rich foods often produce large amounts of AA during thermal processing, so many methods for AA detection have been developed in various fields. GC-MS (Wawrzyniak & Jasiewicz, 2019), LC-MS (Jozinovi´ et al., 2019), and CE (Mollakhalili-Meybodi, Khorshidian, Nematollahi, & Arab, 2021) are all commonly used technologies for detecting AA in food. Due to its low volatility and polarity, AA is determined by GC-MS derivatization with potassium bromate and potassium bromide. However, direct GC-MS analysis of AA has a number of drawbacks: the lack of characteristic ions and matrix interference in the mass spectrum of underivatized AA increased background noise and poor retention on conventional GC columns. LC-MS is now widely used by research institutions in its derivatization-free and sensitive detection of AA, and the method can also be used to simultaneously detect AA and other toxic compounds in a sample (Desmarchelier, Hamel, & Delatour, 2020). In addition, CE has also been applied to detect AA content, in which compounds with different charges can be detected by applying a high voltage that allows them to dissolve in the electrolyte and move at different rates (Mollakhalili-Meybodi et al., 2021). In addition, with the development of the times, classical chromatographic methods have been refined to adapt to more complex analytical conditions. In the analysis of AA in coffee, the cumbersome sample pretreatment leads to signal interference from interfering peaks in detecting AA by LC-MS. In contrast, AA was extracted from coffee brewing samples using dispersive liquid-liquid microextraction (DLLME) and detected by liquid chromatography-high resolution mass spectrometry (LC-HRMS) for the content of AA in coffee, which can effectively exclude the interference of impurities and improve the detection efficiency and accuracy (Galuch et al., 2019). Furthermore, Zhang and colleagues applied UPLC-MS / MS to determine AA in human urine and found higher AA levels in men than in women after a short-term diet, providing methodological support for assessing dietary exposure to AA in the short term (Zhang et al., 2020).
The previously developed chromatographic methods such as cumbersome sample pre-treatment, expensive instrumentation, and high detection costs have many limitations. Hence, the immunoassay has been developed based on antigen-antibody specificity. It is normally possible to cross-link AA with immune-responsive carrier proteins such as BSA and OVA to generate incomplete antigens and then obtain polyclonal antibodies with particular recognition ability by immunising animals since AA lacks antigenic determinants and immunogenicity. The requirement for real-time and rapid detection of AA can be met by immunochromatographic test strips (ICS) (Li et al., 2019; Wu et al., 2019). In addition, as an emerging analytical technology, electrochemical sensing analysis based on biomolecules has been intensively developed in the environment, medicine, and food. Fe3+/hemoglobin electroactive groups are found in each of the four polypeptide chains that make up haemoglobin. When Fe3+ and Fe2+ are reversibly converted from each other, Hb's electroactivity is increased. AA can form a stable adduct AA-Hb with hemoglobin, so the biosensor can modify Hb on the surface of the transducer to detect AA (Asnaashari, Kenari, Farahmandfar, Abnous, & Taghdisi, 2019). Electrochemical sensors can detect AA with high specificity, high sensitivity, and excellent reproducibility because they are not affected by the presence of other food components. Therefore, with the continuous improvement of analytical methods, more superior analytical tools will undoubtedly be applied to the future detection and analysis of AA in food.
TOXIC EFFECTS OF ACRYLAMIDE ON HUMAN HEALTH
Neurotoxicity
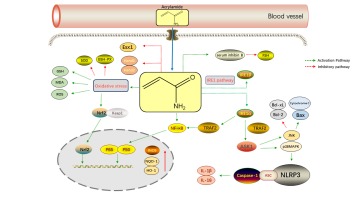
Studies have found that AA causes neurotoxicity and neurodegenerative disorders in experimental animals and humans, causing ataxia (Sui et al., 2020). Neurotoxicity caused by AA in experimental animals might manifest as hind limb dysfunction, skeletal muscle weakness, and weight loss in rats. In humans, on the other hand, AA's neurotoxicity is primarily evidenced by sensory or motor neuropathy, sleepiness, or cerebellar ataxia (Bin-Jumah, Abdel-Fattah, Saied, El-Seedi, & Abdel-Daim, 2021). (Zong et al., 2019) found that five weeks of exposure to AA in rats induced neuroinflammatory response in the brain, manifested by upregulation of IL-1β, IL-6 and IL-18. Microglia are the most common cells in the central nervous system's macrophage population and are involved in a variety of neurodegenerative disorders and neurological traumas. AA has been demonstrated to activate microglia, as evidenced by increased expression of the microglial markers CD11b and CD40. A study by (Nagashima et al., 2019) believes that the brain's hippocampal region may be the site of neurotoxicity produced by the AA. Protein analysis of hippocampal tissue taken from rats after five weeks of gavage revealed a decrease in triosephosphate isomerase, mitochondrial creatine kinase U-type, creatine kinase β-type and proteasome subunit α type-1 activity. (Zhang et al., 2020) also found that AA caused degeneration of rat nerve terminal axons and decreased noradrenergic axon density in rat prefrontal cortex, hippocampus, and posterior pontine cortex . Therefore, AA-induced noradrenergic neuropathy may be a significant cause of cognitive dysfunction caused by AA poisoning in patients. AA-induced neurotoxicity is also closely related to oxidative stress. Because of the lower activity of antioxidant enzymes in the central nervous system than in other tissues, nerve cells are vulnerable to reactive oxygen species (ROS) attacks, causing oxidative damage to brain tissue. AA is a soft reagent that preferentially reacts with soft nucleophiles such as glutathione or sulfhydryl groups in proteins, interfering with cellular redox signalling pathways and causing neuronal damage (Faria et al., 2019). Activation of cellular signalling pathways eventually leads to changes in various signalling regulators. During cellular oxidative stress, AA reduced the activity of SOD and GSH-Px and increased the content of ROS and MDA. NF-E2-related factor 2 (Nrf2) was rapidly dissociated from Kelch-like ECH-associated protein 1 (Keap1) and subsequently translocated to the nucleus. Simultaneously, the genes hemeoxygenase 1 (HO-1) and quinone oxidoreductase 1 (NQO-1) downstream of Nrf2 were also increased in expression in the nucleus. Activation of the Nrf2 signalling pathway attenuated AA-mediated cellular oxidative damage but was insufficient to reverse AA-induced cellular damage (Figure 4) (Pan et al., 2018). Thus, the neurotoxic mechanisms of AA include induction of neuroinflammation, inhibition of key cellular enzymes, damage to nerve cells, inhibition of neurotransmission and reduction of nerve terminal axon density.
Carcinogenicity
Animal experiments have shown that exposure of rats to high concentrations of AA can lead to testicular sphincter tumors and thyroid and mammary fibroadenomas (Jiang et al., 2021). In light of its rodent carcinogenicity, the IARC has classified AA as a potential carcinogen for humans. Nevertheless, the carcinogenicity of AA in humans has been much debate so far (Zhivagui et al., 2019). Giorgia Adani et al. found that AA in foods increased the risk of ovarian and endometrial cancers in premenopausal women (Adani et al., 2020). For studies related to breast cancer incidence, AA was not significantly associated with the risk of breast cancer in the postmenopausal women population. However, exposure to high AA was associated with a significantly increased analysis of the premenopausal women population risk of breast cancer (Adani et al., 2020). Therefore, the carcinogenicity of AA may be related to the secretion of sex hormones. There was no correlation between AA intake and lung cancer incidence in both men and women who participated in a prospective analysis of 85,303 Japanese participants (Liu et al., 2020). Esophageal, gastric, and colorectal cancers were not linked to dietary exposure to AA in this author's previous research (Liu et al., 2019). There were also studies showing that the intake of AA was not associated with renal cell carcinoma (Jiang, Teng, Zhu, & Li, 2020) and pancreatic cancer (Kito et al., 2020). In conclusion, AA in food has not been directly related to human cancers in current studies. We may subsequently explore the correlation and specific molecular mechanisms between AA and human cancers in conjunction with hormone levels to further elucidate the carcinogenic risk of AA.
Reproductive toxicity
According to a study conducted by (Zhu, Wang, Jiao, & Zhang, 2021) AA hindered osteoblast differentiation and increased osteoclast maturation in rat embryos, resulting in inadequate skeletal development, decreased body size, and cartilage deformity in rat offspring. Additionally, AA exposure increased foetal resorption considerably and lowered foetal body weights in mice (Yu et al., 2020). AA also causes ovarian dysfunction in female rats by altering steroid hormone release and causing death in the cell (Aldawood et al., 2020). In a study of 468 male subjects aged over 12 years in the United States, AA exposure was found to cause elevated serum inhibin B levels. A member of the TGF- superfamily, serum inhibin B is found in supporting cells, where high inhibin B may inhibit follicle-stimulating hormone(FSH)secretion and reduce spermatogenesis through negative feedback mechanism (Figure 4) (Chu, Liu, Wang, & Lin, 2003). AA can impair adult male fertility by reducing spermatogenesis and inducing morphological and functional defects in spermatozoa (Ivanski et al., 2020). For females, AA can affect the estradiol level in premenopausal women and influence reproductive function by altering the secretion of sex hormones. Meanwhile, AA can also enter the fetus or newborn through the placental barrier or breastfeeding, affecting the normal development of human offspring. Yu D et al. found that AA exposure reduced the absolute and relative weights of the placenta and embryo, leading to placental atrophy, reduced number of vasa vasorum and incomplete embryonic development (Yu et al., 2019). AA also causes the expression of essential placental genes such as Esx1, bHLH transcription factors Hand1 and Hand2 mRNAs and induce placental cell apoptosis (Figure 4) (Yu et al., 2019). In summary, the mechanisms of AA-induced reproductive toxicity are mainly related to the secretory effects of sex hormones, the placenta's function, and the embryo's development.
Genotoxicity
Studies have shown that AA readily binds to glutathione and sulfhydryl groups in proteins and can increase the concentration of ROS and MDA, inducing cellular oxidative stress, mitochondrial dysfunction and DNA damage, thereby causing genotoxicity (Salimi et al., 2021). In addition, as a toxic metabolite of AA, GA directly attacks DNA molecules and increases DNA reactivity to cause cellular genomic instability in the presence of insufficient glutathione levels. GA is a highly genotoxic chemical that combines with purine bases of DNA in cells and mediates cyto-genotoxicity by promoting micronucleus formation and DNA double-strand breaks in Sertoli cells, according to (Yilmaz, Yildizbayrak, & Aydin, 2020). (Ibrahim & Ibrahem, 2020) found that AA decreases glutathione levels, inhibits SOD activity, and induces oxidative DNA damage to cause liver, kidney, and brain damage in Clarias gariepinus. In addition, Wang B et al. investigated 3,270 adults in Wuhan and found that exposure to AA in adults caused elevated fasting glucose, which may be associated with cellular DNA damage and lipid peroxidation (Wang et al., 2020).
Other toxicity
In addition to the above toxicity, people should be alert to the possibility that AA may cause hepatosplenic toxicity. Donmez DB et al. AA damaged rat liver tissue leading to vascular congestion, cellular infiltration, and necrotic cells (Donmez, Kacar, Bagci, & Sahinturk, 2020). Furthermore, (Yue et al., 2020) found that AA affects liver function in rats by interfering with glucose metabolism, blocking insulin release, and disrupting glucose homeostasis. In addition, AA hepatotoxicity is associated with inflammation, which can damage hepatocytes by releasing large amounts of inflammatory factors and induction of oxidative stress by NLRP3 inflammasomes. The NLRP3 inflammasome is activated by oxidative damage and endoplasmic reticulum stress (ERS). IRE1 interacts with TRAF2 to activate ASK1, then JNK and p38MAPK. The MAPK signalling pathway can activate the NLRP3 inflammasome, and Caspase-1 increases IL-18 and IL-1 production (Bo, Yilin, Chaoyue, Lu, & Yuan, 2020). JNK stimulates the expression of pro-apoptotic proteins Bax and cytochrome c while inhibiting anti-apoptotic proteins Bcl-2 and Bcl-xL. IRE1 boosts the NF-B signalling pathway and causes NF-B nuclear translocation, as well as INOS and NO generation (Figure 4) (Sun, Qu, Wang, Shao, & Sun, 2018). In addition, (Komoike, Nomura-Komoike, & Matsuoka, 2020) found that AA can cause splenic toxicity. They exposed adult zebrafish to AA, and after one month, their spleen was severely swollen with symptoms such as haemorrhage and inflammation (Komoike et al., 2020). The latest toxicity studies of acrylamide in vivo are summarized in Table 1. Based on the potential health hazards of AA in humans, there is a growing interest in the degradation of AA and toxic metabolites in current studies. (Zilli et al., 2021) found that UV could degrade AA with hydrogen peroxide (H2O2) into less toxic by-products. The researchers found that microalgae Desmodesmus quadricauda are very sensitive to the presence of contaminants such as acrylamide. They irradiated a solution containing AA (1.5 mg/L) in H2O2 (0.034 g/L) with UV for 10 min and then used the solution to culture D. quadricauda. The growth rate of D. quadricauda was almost unaffected, and the absence of AA in the solution was detected by HPLC (Zilli et al., 2021). This suggests that we can develop more efficient methods to reduce the toxicity of AA in the future and ensure people's health safety.
Table 1
Toxic effects of acrylamide in vivo
RISK ASSESSMENT OF DIETARY EXPOSURE TO ACRYLAMIDE
As researchers continue to delve into the toxic effects of AA, we wonder how much AA is consumed daily from food or the environment. AA's risk assessments have found that people are inevitably exposed to AA's toxic substance in their daily lives, including adults and children. For the literature review in the last three years, we examined some of the literature reporting people's intake of AA daily. In the 2002 FAO/WHO report, the general population's daily dietary exposure to AA was established to be 0.3-0.8 μg/kg (Andačić et al., 2020). (Basaran & Aydin, 2020) found that among 263 Turkish people, the average daily AA exposure was 0.11 ± 0.084 µg/kg for coffee drinking consumers and 0.057 ± 0.037 µg/kg for non-coffee drinking consumers, with an average daily exposure of 0.10 ± 0.065 µg/kg for men and 0.12 ± 0.097 µg/kg for women. According to the findings, it is possible that the typical intake of Turkish coffee (small size due to the cup size) will restrict individual exposure to AA in the long term. The consumption of AA is reduced as a result of factors such as the type of coffee, how it is processed, and where it is grown, which is a positive development (Basaran et al., 2020). The USFDA assessed the daily dietary exposures of the US population to AA in food for those >2 years old and those 2 years old based on 2011-2015 data. The average daily dietary exposure to AA was 0.36 µg/kg for those >2 years old, and 1.42 µg/kg for those 2 years old, with cereal-based infant cereals and infant cookies accounting for 27% of AA intake. Additionally, the JECFA estimates that the average adult's daily dietary consumption of AA is between 0.2 and 1.0 µg/kg. According to JECFA, children consume twice as much AA than adult consumers, in part because children consume more AA-containing foods (e.g., chips, fries, and cookies) and have a lower average body weight than adults (Abt et al., 2019). In Japan, a study involving 240 participants (aged 40 to 74 years) from five locations (Yokote, Saku, Chikusei, Murakami, and Uonuma) discovered that men had an average daily AA intake of 0.27 µg/kg while women had an average daily intake of 0.32 µg/kg (Kito et al., 2020). In addition to the effect of dietary habits, Goerke K and colleagues studied twenty healthy non-smoking Caucasian volunteers (21-31 years). It was assigned to two groups: ten (half of each sex) who did not declare any dietary preference and ten (half of each sex) who followed a vegan lifestyle by abstaining from animal products. The researchers found that the daily dietary AA exposure for all volunteers during the 10-day study was 0.32 ± 0.19 µg/kg, with an intake of 0.29 ± 0.18 µg/kg for men and 0.35 ± 0.20 µg/kg for women. The vegan volunteers had a higher AA intake of 0.38 ± 0.23 µg/kg than the omnivorous volunteers at 0.26 ±0.10 µg/kg (Goerke et al., 2019). This suggests that a vegan lifestyle may be more susceptible to AA exposure under certain circumstances, especially when consuming thermally processed vegan foods such as meat substitutes.
In addition, AA dietary exposure is a significant safety risk for children in their developmental years. (Mesias, Delgado-Andrade, Holgado, & Morales, 2020) found that children are most exposed to AA through diet. By detecting potato chips in 31 elementary school cafeterias in Spain, the average AA level was 329 µg/kg (Mesias et al., 2020). On average, semi-industrial sangak bread contained 135 grammes of AA per kilogramme, making it the most dangerous for youngsters to consume (Eslamizad et al., 2019). In addition, the researchers collected 143 different meals from companies providing school canteens in the Umbria region of central Italy. When it came to school-age children's AA consumption, the researchers found that the mean value for potatoes and bakery items was 2.16 µg/kg, while the highest values were found to be in meat (222 µg/kg) and the egg products were 1.51 µg/kg (Branciari et al., 2020). Therefore, dietary exposure to AA due to school cafeteria food should also be considered a health concern and taken seriously. The ANSES recommends an AA benchmark value of 0.2 µg/kg for children under three years of age, while a survey of 705 French children under three years of age found an average daily exposure of 0.141 µg/kg for children aged 1-4 months and 0.708 µg/kg for children aged 13-36 months. Extrapolating from age classes, 26% to 98% of children exceeded the baseline value of 0.2 µg/kg, which may be related to the initiation of exposure to potato products and cookies in children aged one year (Sirot, Rivière, & Leconte, 2019). In addition, a study by Hsu CN et al. noted that the higher the concentration of AA detected in the urine of children with chronic kidney disease, the greater the likelihood of cardiovascular disease, indicating that excessive intake of AA in children may lead to exacerbation of the disease or lesions of other organs (Hsu et al., 2020). Table 2 summarizes the daily intake of AA in specific national populations, providing a more intuitive understanding for assessing AA dietary exposure. Thus, for the risk assessment of AA, we found that the dietary intake of AA in children is at least twice as high as that of adults, and in the future, researchers should pay more attention to the dietary exposure to AA and find ways to reduce the AA content in foods to ensure people's food safety.
Table 2
Dietary exposure to acrylamide (µg/kg) through food consumption
MEASURES TO REDUCE ACRYLAMIDE IN FOOD
Application of plant antioxidants
Based on the toxic effects of AA on human health, we should take the initiative to find more techniques and methods to reduce the formation of AA in food and reduce the health risk of people. It was found that some antioxidant substances exist in natural plants to reduce AA formation. (Wang, Ji, Ji, & Chen, 2019) found that Blueberry anthocyanins extract (BAE) inhibited the formation of the GA and reduced the accumulation of GA-DNA adducts in the kidney, liver, and lung of rats. (Yu, Chen, Meng, & Chen, 2020) found that To prevent the generation of AA, lipophilic grape seed proanthocyanidin (LGSP) reduced lipid oxidation in the acrolein pathway and Schiff base levels in the asparagine process (Yang et al., 2021) also found that the polyphenols of apple root bark glycoside phlorizin in apple significantly reduced the amount of AA in French fries after soaking potato fries and preserved the color and flavour of the food itself. There are no examples of antioxidant applications for AA protection and whether we should enhance the development of new plant antioxidant-based drugs or nutraceuticals related to protecting against AA contaminants.
Application of asparaginase
Asparagine is one of the precursors for AA formation, and the asparagine content in food ingredients directly affects AA production. Because asparaginase is stable in food and does not cause toxic reactions in humans, asparaginase can be added to food as a food additive to reduce the amount of AA in food (Cunha et al., 2019). Wang Y et al. found that a novel recombinant Asparaginase produced by Palaeococcus ferrophilus retained more than 98% of its initial activity after three months of storage at 4°C. In addition, enzyme treatment of French fries (10 U / mL, 85°C, 15 min) resulted in a significant 79% reduction in AA content compared to untreated fries (Wang, Wu, Zhang, Xu, & Mu, 2021).
Optimization of baking conditions
In the process of baking food, roasting parameters can affect the formation of AA. (Bertuzzi, Martinelli, Mulazzi, & Rastelli, 2020) found that baking Arabica and Robusta varieties at an elevated temperature from 90 °C to 215 °C resulted in a maximum AA content when the temperature reached 175-177 °C after 10 min of baking. However, when the temperature reached 203-205°C after 14 min, the AA content decreased rapidly, along with decreased asparagine and reduced sugar content (Bertuzzi et al., 2020). AA is produced above 120°C during frying and baking but undergoes degradation at temperatures above 180°C (Esposito et al., 2020). Therefore, we should determine the appropriate baking temperature and time to reduce the AA content during food processing. In addition, the moisture content of food ingredients also affects the production of AA. For example, potatoes, coffee, and cereals with low water content (<5% moisture content) are more favourable for AA formation (Andačić et al., 2020). Food ingredients with pH between 7-8 are more likely to form AA, while changes in the pH of processed foods may result in changes in the content of AA. These changes in baking parameters undoubtedly increase the food safety risks for people, so accurate control of food baking conditions is also an effective measure to reduce the production of AA.
CONCLUSION AND FUTURE PROSPECTS
The exposure of AA in food is an unavoidable public health problem for humans. AA can cause neurotoxicity, carcinogenicity, reproductive toxicity, genotoxicity and hepatosplenic toxicity, detrimental to human health. Although there is controversy in current research about whether dietary exposure to AA increases the risk of cancer in humans, animal studies have shown that high levels of AA can lead to an increased risk of cancer, suggesting that we should be more vigilant about dietary exposure to AA in humans. In addition, with the development of modern analytical techniques, we can detect AA and its metabolites in food or human urine by LC-MS/MS, GC-MS and CE. However, the detection of AA and its metabolites is limited in routine and clinical applications due to the expensive analytical instruments, high cost and tedious sample handling. Therefore, novel assays that are rapid, inexpensive, and accurate also need to be developed. Risk assessment of human exposure to AA has revealed that with the improvement of quality of life and the introduction of baked goods such as French fries, cookies and bread into people's daily life, adults and children have been exposed to the risk of AA, especially for children, whose bodies are still developing and more vulnerable to the toxic effects of AA. Therefore, it is also crucial to find effective mitigation measures to reduce the formation of AA in food. Government authorities should enact relevant laws and regulations to set minimum residue limits of chemical contaminants AA in food, and food manufacturers should take responsibility to optimize the process conditions to reduce AA production in food. In the future, we should also conduct more research to explore the toxic effects of AA and elucidate the specific molecular mechanisms of AA poisoning, to prove the correlation between AA and human diseases from clinical data, and to establish effective detection methods and mitigation measures to protect people's health and food safety.
Abbreviations
AA; acrylamide
GA; glycidamide
3-APA; 3-aminopropionamide
NOCs; N-nitroso compounds
HAAs; heterocyclic aromatic amines
Hb; hemoglobin
GST; glutathione-S-transferase
PAHs; polycyclic aromatic hydrocarbons
3-MCPDE; 3-monochloropropane-1,2-diol esters
GAMA; N-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-L-cysteine
iso-GAMA; N-acetyl-S-(1-carbamoyl-2-hydroxyethyl)-cysteine
AAMA; N-acetyl-S-(2-carbamoylethyl)-cysteine
SNFA; Swedish National Food Agency
GC-MS; gas chromatography-mass spectrometry
LC-MS; liquid chromatography-mass spectrometry
CE; capillary electrophoresis
DLLME; dispersive liquid-liquid microextraction
LC-HRMS; liquid chromatography-high resolution mass spectrometry
BSA; bovine serum albumin
OVA; ovalbumin
ICS; immunochromatographic test strips
ROS; reactive oxygen species
SOD; Superoxide dismutase
GSH-PX; Glutathione peroxidase
MDA; malondialdehyde
Nrf2; nuclear factor erythroid 2-related factor 2
HO-1; heme oxygenase 1
Keap1; Kelch- like ECH-associating protein 1
NQO-1; NAD(P)H Quinone Dehydrogenase 1
IARC; International Agency for Research on Cancer
FDA; Food and Drug Administration
JECFA; Joint Expert Committee on Food Additives
FSH; follicle-stimulating hormone
Esx1; Extra-embryonic tissue-spermatogenesis-homeobox gene 1
bHLH; basic Helix-Loop-Helix
NO; nitric oxide
ERS;; endoplasmic reticulum stress
JNK c-Jun N-terminal kinase
ASK1; activate apoptosis signal-regulated kinase 1
Bax; BCL2-Associated X
Bcl-2; B-cell lymphoma-2
Bcl-xL; B-cell lymphoma-xL
TNF; Tumor necrosis factor
TRAF2; Tumor necrosis factor receptor associated factor 2
INOS; Inducible nitric oxide synthase
FAO; Food and Agriculture Organization
WHO; World Health Organization
BAE; Blueberry anthocyanins extract
LGSP; lipophilic grape seed proanthocyanidin
Author contributions
Tingdong Yan and Yi Cai created the research concept and design. Chaoming Huang, Shisheng Jiang, and Yantianyu Yang collected and/or assembled data. Yantianyu Yang, Shuhan Gao, Zihan Lin, and Wei Gu analysed and interpreted the data. Chaoming Huang wrote the article. Tingdong Yan and Yi Cai contributed to the article's revision. Tingdong Yan and Yi Cai gave final approval to the article.