INTRODUCTION
Endophytic fungi are inhabiting plant tissues without triggering evident harmful effects (Adeleke & Babalola, 2021; Griffin, Harrison, Mccormick, Burghardt, & Parker, 2019). They have a key function in plant growth and health (Bhardwaj, Sharma, Jadon, & Agrawal, 2015). Endophytes in general produce different types of metabolites and sometimes can produce similar bioactive components synthesised by host plants (Sharma, Dhankar, Thakur, Raza, & Katare, 2016). Studies have shown the capacity of endophytes to manufacture varieties of bioactive molecules with remarkable biological activities including antioxidant, anticancer, antivirus, antituberculosis, antiparasite, immunomodulatory and insecticides among others (Toghueo, 2020; Uzor, Osadebe, & Nwodo, 2017). This ability has prompted scientists to investigate endophytes as an complementary source of plant pharmaceuticals (Subban, Subramani, Srinivasan, Johnpaul, & Chelliah, 2019).
Medicinal plants are found to harbor endophytes that are capable to synthesize bioactive agents with potential biological activity. Moreover, it has been established that about 18% of plant-derived metabolites are also produced from the fungi inhabited their tissues (Chowdhary & Kaushik, 2015). The best example is Taxol (anticancer drug) which was isolated from Taxus brevifolia and its associated fungus Taxomyces andreanae (Stierle, Strobel, & Stierle, 1993). There are some studies reporting the isolation of endophytic fungi from plants used in Sudan traditional medicine. For example, Mahdi, Mohamed, and Yagi (2014) obtained 17 isolates from Datura stramonium, Moringa oleifera and Prosopis chilensis with remarkable antimicrobial activity. Moreover, Byssochlamys spectabilis and Alternaria sp., obtained from Euphorbia prostrata, revealed respectively potent antitumor and antimicrobial activities (Khiralla et al., 2016). Curvularia papendorfii isolated from Vernonia amygdalina was found to possess potent antiviral effect. Moreover, kheiric acid which was isolated from this endophytic fungi was shown to exert high antifungal activity (Khiralla et al., 2020).
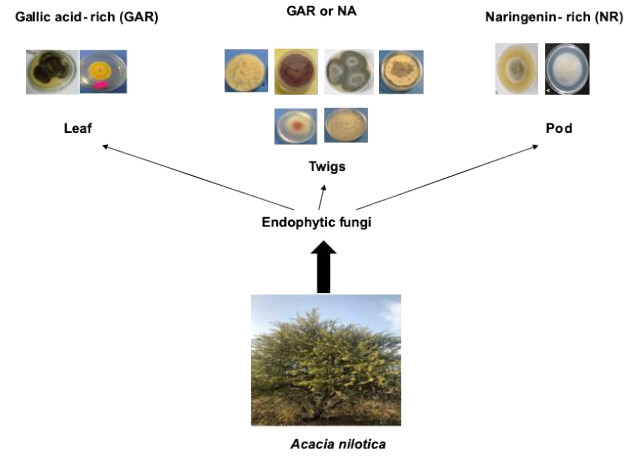
Acacia nilotica (L.) Delile (family Fabaceae) is a plants used traditionally in Sudan to cure cold and flu, pharyngitis, hypertension, stomachache, malaria, fever, wounds, furuncles, pustule and as antiseptic (Yagi & Yagi, 2021). The plant is rich in phenols, saponins, triterpenes and sterols (Rather, Shahid-Ul-Islam, & F, 2015). Pharmacologically, A. nilotica was found to possess antiplasmodial, antibacterial, antifungal, antiviral, antioxidant, antidiarrhoeal, antimutagenic, antihypertensive, analgesic, antipyretic, galactagogue, hemolytic, hyperglycaemic, antidiabetic abortifacient and anti-infertility activities (Rather et al., 2015). Few studies have identified endophytic fungi resident inside the tissues of A. nilotica and evaluated their potential biological activities. Meenambiga and Rajagopal (2016) isolated 553 endophytic fungi isolates from A. nilotica grown in India. They found that the endophyte Eupenicillium sp had a high antimicrobial activity against Streptococccus mutans and Candida albicans. Singh and Kaur (2016) isolated thirty-six endophytic fungi from A. nilotica, endogenous to India too, and they found that the endophytic fungus Aspergillus awamori exerted antidiabetic property. Recently, Shaikh, Khan, and Baig (2021) isolated 26 endophytic fungal species. They also noted that the same species could host different population of endophytic fungi which could be attributed to many factors including geographical location of the plant, and different ecological conditions (Griffin et al., 2019). Hence, the present study aimed to isolate endophytic fungi from leaf, twig and fruit of A. nilotica indigenous to Sudan and to determine their phenolic composition, antimicrobial and antiradical properties. Results were compared with extracts from the host plant organs.
MATERIALS AND METHODS
Plant materials
Leaves, twigs and fruits samples of A. nilotica were randomly harvested from a tree grown in Khartoum (15°38′ N 32°32′ E), Central Sudan. Plant samples were processed according to the guidelines of Nalini et al. (2005).
Isolation, purification and preservation of endophytic fungi
Endophytes were isolated from collected organs, after surface sterilization, following the procedure given by Zhang, Zhou, and Yu (2009). Samples were successively rinsed under running tap water and distilled water. Then, with sterile razor blades, they were cut into small pieces (2–4 mm). Segments were sequentially immersed in 70% EtOH for 60 seconds, 0.5% NaOCl for 5 minutes, 70% EtOH for 30 seconds and finally dipped in sterilized distilled water for 5 min. The aseptically air-dried segments were placed on Petri dishes (3/plate) containing potato dextrose agar (PDA) impregnated with 0.01% gentamycin. The well-sealed plates were incubated for 5–7 days at 27 °C. Then after incubation, hyphal tips were transferred onto a fresh PDA medium. The process was repeated until getting pure isolates. The purified isolates were then kept on slants of PDA medium at 4°C or in 15% (v/v) glycerol stock solution for spores and mycelium at – 20° C.
Extracts preparation from endophytic fungi and host plant
Fungal isolates were subjected for large scale biomass production as described by (Campos et al., 2008). Each isolate was inoculated in 30 Petri dishes containing PDA medium. Fungal biomass, including the medium, were crushed and macerated in methanol (500 mL) for 6 days, then filtered and filtrates were evaporated to dryness. For host plant extracts, 100 g of powdered dried fruit, leaf and twig of A. nilotica were soaked, separately, in 500 mL of methanol following the same procedure. All extracts were weighed and kept in well closed bottles at 4º C.
Total polyphenols, flavonoids and tannins contents
Total polyphenols, flavonoids and tannins contents were performed as described by Wolfe, Wu, and Liu (2003),Ordonez, Gomez, and Vattuone (2006) and Sun et al. (1998) respectively. Details were given in supplementary file (Appendix A ).
Chemical profile
Analysis of phenolic profile of extracts was performed by HPLC-DAD system (Shimadzu Scientific Instruments, Kyoto, Japan) as described by Movahhedin et al. (2016). The stationery phase was Eclipse XDB C-18 reversed phase column and it was set up at 30 °C. Identity and concentration of compounds were achieved by comparison with standards. Details were given in supplementary file (Appendix A ).
Antimicrobial activity
Antibacterial and antifungal activities were performed by the method described by Mbaveng et al. (2008) and M Mothana and Lindequist (2005) respectively. Details were given in supplementary file (Appendix A ).
Radical scavenging activity
The antiradical activity of extracts was determined by the 1, 1-diphenyl-2-picryl hydrazyl (DPPH) assay (Zengin et al., 2015). Details were given in supplementary file (Appendix A ).
RESULTS AND DISCUSSION
Figure 1 shows cultures of the 10 endophytic fungal isolates which were obtained from the twig (6), leaf (2) and fruit (2) of A. nilotica grown in Sudan.
Figure 1
Fungal endophyte cultures: a, (L1) Chaetomium sp; b, (L2) Emericella sp.1; c, (F1) Alternaria sp; d, (F2) Mucor sp; e, (T1) Aspergillus sp; f, (T2) Chrysosporium sp; g, (T3) Curvularia sp; h, (T4) Emericella sp. 2; i, (T5) Fusarium sp; j,(T6) Phoma sp.
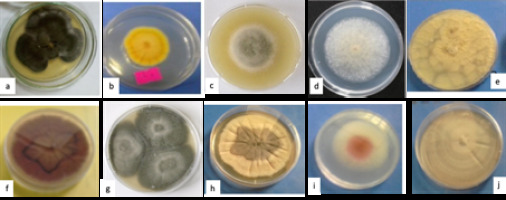
Table 1
Antimicrobial activity of methanolic extracts of endophytic fungi isolates and organs of host plant (A. nilotica).
Table 2
Scavenging radical activity and total polyphenolic, flavonoids and tannins contents of methanolic extracts of endophytic fungi isolates and organs of host plant (A. nilotica).
Table 3
Phenolic profile (μg g−1) of methanolic extracts of endophytic fungi isolates and organs of host plant (A. nilotica) by HPLC analysis.
Antimicrobial activity
Antimicrobial resistance has become a global concern and the explore for original natural antimicrobial molecules is imperative (Dhingra et al., 2020). The antimicrobial activity of endophytic fungi and host organs extracts was examined and results are given in Table 1. Extracts of endophytic fungi showed a broad spectrum of antimicrobial activity. S. aureus was more susceptible to the studied endophytic fungi extracts where 70% (7/10) of fungal endophytic extracts exhibited antibacterial activity with inhibition zones ranged 12 – 15.5 mm. Isolate F2 extract displayed the highest activity. The same endophyte gave the highest inhibition zone (14 mm) against E. coli. Extracts were less active against B. subtilis and P. aeruginosa where the highest activity was recorded from extracts of isolates F1 (12 mm) and L1 (11.7 mm) respectively. Extracts of three isolates namely; T3, L1 and F1 exerted high antifungal activity (20.6, 20 and 18.3 mm respectively) against A. niger, even higher than those obtained from the standard drug Nystatin (16 mm) and the host organs extracts (11.3 – 13.3 mm). The latter (isolate F1) showed the best antifungal effect against C. albicans (22.7 mm) but with lower inhibition zone compared to that exerted by the standard drug (30 mm) and leaf and twig extracts of the host plant (31.3 and 23.3 mm respectively). Variation in sensitivity of tested microorganisms towards different endophytic fungi could be due to nature of isolates and metabolites present in extracts beside their mechanism of action on pathogens (Barbour et al., 2004).
Free radical scavenging activity
Natural antioxidants from plants and microorganism have been known to act as potent preventive therapy for treating reactive oxygen species related to various ailments like diabetes mellitus, cardiovascular and neurodegenerative diseases and aging (Sujatha & Asokan, 2017). The antiradical activity of the isolated endophytic fungi and host organs extracts were evaluated for their capacity to scavenge the DPPH radical. Results are depicted in Table 2. Only two endophytic fungi; isolates T4 and T5 revealed considerable antiradical activity with inhibition values of 77.9% and 69.5% respectively and IC50 values of 302 and 478 µg/ml respectively. All other extracts of endophytic fungi showed weak antiradical activity (˂ 50%). On the other hand, the three host organs extracts exerted potent antiradical activity (91.5% — 93.6% and IC50 14 – 57 µg/mL). Nevertheless, it would be necessary in the future to carry out more complementary assays to all endophytic extracts in order to understand in depth their antioxidant properties.
Total phenolic contents
The total polyphenolic content of endophytic fungi extracts was in the range from 15.9 to 66.68 mg GAE/g with the highest value been recorded for isolate T4 extract. The total flavonoids content ranged from 2.63 to 16.44 mg QE/g with the highest amount found in isolate T5 extract. The total tannins content was in the range of 35.12 to 58.85 mg TAE/g where isolate F1 extract scored the highest content (Table 2 ). Comparing these values with those obtained from the three host organs it was clear that endophytic fungi methanolic extracts accumulated lower content of total polyphenolic and flavonoids than the host organs while some endophytes had comparable values of total tannins content to those of the leaf and fruit of host but lower values than that of the twig. Many studies correlate the antioxidant activity of extract to its phenolic content (Farag, Abdel-Latif, Baky, & Tawfeek, 2020; Wong, Xiao, Wang, Ee, & Chai, 2020). In this study it was seen that extracts of the isolates T4 and T5 which exerted the highest antiradicals activity had also the highest polyphenolic content.
Phenolic profile
Phenolic profiles of methanolic extracts from isolates of endophytic fungi and host plant organs were determined using HPLC technique (Table 3 ). Chromatograms are shown in a supplementary file. The results obtained revealed peaks at different retention times, which were compared to the retention times of the standards (11 compounds) used. Methanolic extracts of endophytic fungi were dominated by two compounds, gallic acid and naringenin. Gallic acid was detected in extracts of the two endophytic fungi (isolates L2 and L1) isolated from the leaf as well as isolates T4 and T2 (both isolated from the twig). Naringenin was found in the extracts of isolates F1 and F2, which were isolated from the fruit, as well as four endophytic fungi (isolates T1, T3, T5 and T6) isolated from the twig. Interestingly, it was observed that the extracts of endophytic fungi which contained naringenin were devoid from gallic acid and the opposite was true. Accordingly, the fungal endophytes could be grouped into gallic acid-rich endophytic fungi and naringenin-rich ones. Moreover, this negative correlation was not detected in the extracts of host organs where the two compounds (gallic acid and naringenin) coexist and identified in all extracts. Caffeic acid and dihydroxy flavone which were not detected in the host organs were found in all endophytic fungi except one isolate for the former and 2 isolates for the latter. In contrary, syringic acid which was accumulated in abundance in the host organ was not detected in all endophytic fungi extracts. Ferulic acid was found only in isolate F1 extract and was not identified in all other endophytic and host organs extracts. Also the endophytic fungi isolated from twig had higher relative abundance in gallic acid and naringenin than those isolated from the leaf or fruit. The high antiradical activity of isolates T4 and T5 extracts could be attributed to the highest accumulation of gallic acid and naringenin respectively. Many studies have proven the antiradical properties of these two phenolic compounds (Badhani, Sharma, & Kakka, 2015; Rashmi, Magesh, Ramkumar, Suryanarayanan, & Subbarao, 2018; Zheng, Deng, Guo, Chen, & Fu, 2019). Naringenin could also partially participated to antibacterial activity of isolate F2 extract (Agus, Achmadi, & Mubarik, 2017).
CONCLUSION
Ten endophytic fungal isolates were obtained from the leaf, fruit and twig of A. nilotica tree grown in central Sudan. Nine of the isolates belong to Ascomycota and only one belongs to Zygomycotina. Some isolates revealed significant antimicrobial and antiradical activity, in addition, they contained considerable amount of phenolics and hence could be an alternative source for biomolecules with multiple industrial applications. Although the number of isolates is not significant to draw conclusive remarks, nevertheless, it was observed that endophytic fungi isolated from the leaf accumulated gallic acid while those from the fruit tend to have naringenin and those isolated from the twig could have either gallic acid or naringenin (but not together). Therefore, it would be interesting to understand the factors influencing the synthesis of metabolites by endophytes and the integrated metabolism of the plant-endophyte relationship. The present study also demonstrated that endophytes could be a key approach to search for bioactive molecules with interesting pharmaceutical applications.