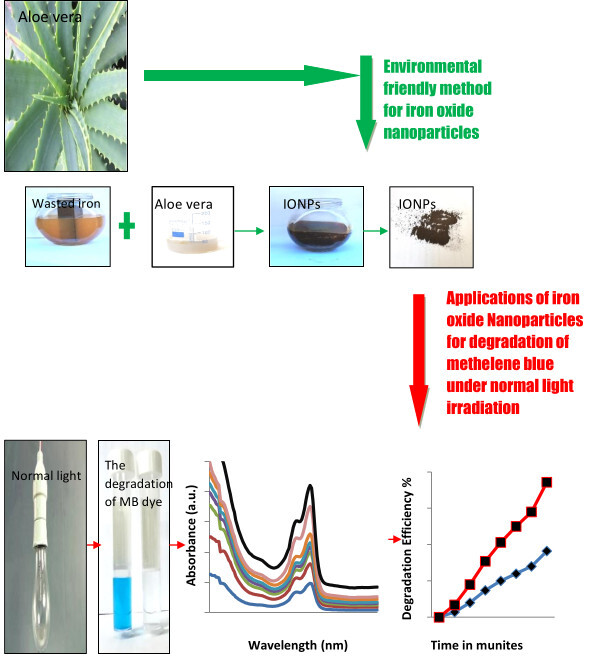
Introduction
The term "nanoparticles" (NPs) refers to small, inorganic or organic particles having a diameter of less than 100 nm that have unique features when compared to bulk materials (Xue, Tang, Qi, Wei, & Ru, 2021). In comparison to other materials, (Herlekar, Barve, & Kumar, 2014) iron oxide (IONPs) comprise required materials with significant future advantages (Herlekar et al., 2014), different physical qualities such as low toxicity, powerful catalytic activity, small size, and a variety of physical properties (Cornell & Schwertmann, 2006; Guo, Wang, Tjiu, Pan, & Liu, 2012; Issa, Obaidat, Albiss, & Haik, 2013). Human activity is now connected with chemicals in many aspects of life, and these compounds are damaging to the human body. Notably, some such compounds are continuously utilized, which is acknowledged as not being a "completely safe chemical and not in return." There is a substance that is extremely hazardous. Chemists in the Middle Ages were affected by the damage to explosive and toxic substances, and severe environmental problems began in the 17th century as a result of mining cinder released in Germany, and pigment and other chemicals from carbon tar were released in the 18th century, leading to mine discharge. The overwhelming usage of iron metal led to the foundation of a waste yard of a thousand tons of rusty scrap and rubbish without any resolution to reuse it or take advantage of it until today, causing a dangerous environmental issue in various places in the world. In the summer, the iron consumed and the enormous quantities thereof constituted an environmental burden and have become a significant disposal difficulty. The MB dyes are among the many organic pollutants of water sources, and the reason for this is because they are of great importance and have wide use in various industries, as they are used in the textile industries, in printing, in photographic colors, as additives in the petroleum industries, etc. (Aliasghari et al., 2021; Maleki, Pajootan, & Hayati, 2015; Ren, Rahmani, Meletis, Zhang, & Cao, 2021; Rominiyi et al., 2020; Taghipour, 2004). As a result, the presence of MB dye in wastewater is both undesirable and illegal, thus it is advisable to remove these components from the water before reintroducing them into the environment. This might be related to the aesthetics of the surroundings, the toxicity of pigments, and the long-term consequences on the environment and human body (Bedin, Martins, Cazetta, Pezoti, & Almeida, 2016; Djilani et al., 2015; Zhao, He, Chen, & Hu, 2018). Economic efficiencies, nuclear economy, simplicity, benignity, non-toxicity, elimination of poisonous and harmful chemicals, ease of availability, and removal of ecologically hazardous elements make the green synthesis of the IONPs employed in biological components by plant extracts a major issue (Chamorro et al., 2020; Fallah, Farzaneh, Yousefzadi, Ghorbanpour, & Mirjalili, 2019; Yew et al., 2018). For millennia, people have recognized and used the aloe vera plant for its health, beauty, and skin benefits. The name Aloe vera is derived from the Arabic word "Alloeh," which means "shining bitter substance," and the Latin word vera," meaning "true." 2000 years ago, Greek scientists believed that aloe vera was a universal cure-all. The Egyptians referred to aloe as "the plant of immortality." Aloe vera is being used in a range of environmental therapies. Aloe vera Burm F., also known scientifically as Aloe barbadensis Miller, is a xerophytic plant with succulent leaves. The leaves are divided into three layers: a fleshy inner layer, a thin middle layer, and a thick protective outer layer. The outside layer is composed mostly of cellulose, the middle layer is dominated by barbaloin (aloin A and B), and the inner gel is composed primarily of acemannan, an acetylated glucomannan (Rahmani et al., 2020). The inner gel also contains carbohydrates, anthraquinones, amino acids, vitamins, and proteins (Kato, 2011; Roy & Das, 2015). Several previous studies have employed aloe vera gel to generate antibacterial edible films (Sharma, Verma, Mukhopadhyay, Gupta, & Gupta, 2022), aloe-cellulose composite membranes (El-Baky et al., 2016), and antibacterial nonwoven textiles (Ghayempour, Montazer, & Rad, 2016). Additionally, the function of acemannon in the creation of nanoscaffolds with antibacterial activity is described. Among the most important types of metal oxide nanoparticles are ferric oxide nanoparticles (FONPs), which have magnetic characteristics, biocompatibility, and a high surface-to-volume ratio (Khatami, 2019). As a result, they are used in a range of environmental applications, including catalysis and the removal of heavy metals, antibiotics, and other colors from wastewater (Samira & Carvalho, 2017).
This work is examined in the literature: In (T & Reihaneh, 2021) study, an environmentally friendly technology was successfully used to create superparamagnetic (SPIONs), which were shown to have modest ranges of sizes. The production and characterization of IONPs from iron salts in the aqueous extracts of flaxseed and aloe vera leaves, which were accomplished using a green co-precipitation approach, are included in the paper (Yin & Xu, 2020). The in situ azide alkyne cycloaddition process and the coupling reaction were used to examine the catalytic activity, and the reaction's temperature, solvent, and time were tuned. A synthesis of IONP and its application in cancer treatments studied in Arsalani, Guidelli, Araujo, Bruno, and Baffa (2018); Popescu et al. (2020). The synthesized NPs used rosemary extract and cancer therapies by (Mahanty et al., 2019). In (2019), Cittrarasu, V. and others, Synthesis of Plant Extract Metal Nanoparticles and Application of MB Degradation (Cittrarasu et al., 2019). (Nadeem et al., 2018) describe the green synthesis of metal nanoparticles from plant extract and their application in photocatalytic activity. A research team has invested in these waste materials, including rusty iron and aloe vera extract, which constitute a significant proportion globally. To remove the methylene blue dye in new research that has not yet been covered by any research group. The innovation of making use, without cost to manufacture value and valuable NP materials, of all the materials employed (which are first used as the authors' expertise). Furthermore, neither raw ingredients nor the final product are employed in this research. Without a supplement, all components used are natural. They were recycled into a very effective chemical to eliminate poisons and colors (MB dye). The research was carried out to mix iron scrap rust with aloe vera extract at (300 and 600) oC over 2 hours. IONPs were generated in this section. Treatment of environmental contaminants from MB dye under average light radiation using IONPs. (Fe3O4 and Fe942O) were characterized via X-ray diffraction (XRD) and filed emission scanning electron scanning microscopy (FESEM). Further, the FTIR spectrum is used to determine the vibrations of mode and group functions. For the first time, researchers aim to synthesize IONPs with other plant extracts, but in this work, they utilize aloe vera gel extracts with rust iron. Which is the first time it is used as the author's acknowledgement. The photocatalytic activity of the produced nanoparticles was also tested for MB dye degradation.
Materials and Methods
Experimental work
Our hypothesis is that several factors work together to determine the NPs synthesis, including plant source, extract, temperature, and time of reaction. The plant listed below is represented by Aloe vera Gel. This plant is rich in phenolic substances, especially quercetin, cardiac glycosides, phenolic acids, sulfur, compounds (allicin), alkaloids, flavonoids, terpenes, steroids, vitamins, and minerals. It is very rich in quercetin and fructose, which can reduce ions to NPs because of the presence of vitamin C in these plant extracts. As a first step, the plants were purchased at a market in Baghdad, Iraq.
Preparation of the aloe vera extract
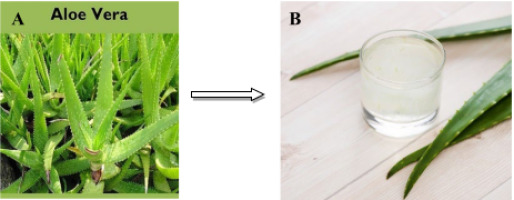
Fresh aloe vera gel has been used in this study. 10 g of plants have been rinsed using filtered water to get rid of any dirt or dust, before being chopped up and blended into a fine powder. The plants were cooked in 100 mL of "distilled water" at 70 oC for 60 minutes. After that, we ran it through Whatman No. 4 filter paper (pore size 20 m) to remove any remaining debris. We then centrifuged the solution at "2000 rpm" for "10 minutes" to remove any remaining debris. Soon after being filtered, the plant extracts were employed in the green synthesis of NPs. Instructions for transforming raw plant material into an extract are provided in figure 1.
Preparation of the rust iron extract
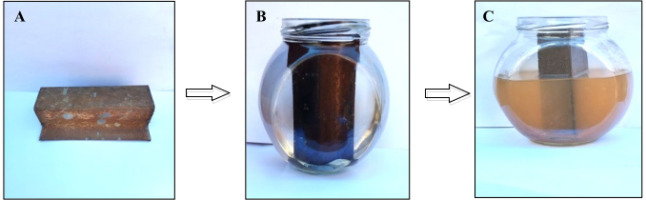
We take 148 g of iron rust to production IONPs. The iron scrap was cleaned with distilled water to remove the contaminants associated with it. The rust iron piece was then placed in a glass container with 500 ml of distilled water and exposed to the sun for five days. The red-colored, 250 ml sized rust iron extract was then collected and kept in sealed tubes for the manufacture of IO. Figure 2 (a–c) shows rust iron extract is created by combining distilled water with rust iron species.
Preparation of (Fe3O4 and Fe902O from the aloe vera extract and rust iron extract
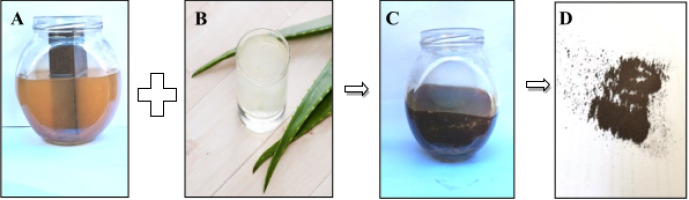
This is created of IONPs by mixing 100 ml of aloe vera with 250 mL from a rusty iron extractor. The solution was then heated to 70°C on the hotplate stirrer for 60 minutes. It notices that, during synthesis, the color of the exhaust reaction quickly changed from transparent off-white to black and alludes to the formation of IONPs. The outcome was resolved at room temperature. To create the IONPs solution, 30 ml of IONPs were placed on a ceramic vine and heated to 200°C for 2 hours. Finally, sealed serum tubes were used to retain NP-solves of IO for further diagnosis, as shown in figure 3.
IONP (Fe3O4 and Fe902O characterization was generated using an extract (aloe vera
The specimen was determined using an XRD analysis of the results provided by the Joint Powder Diffraction Standards Committee (JCPDS) card. A step-by-step test model (XRD-6000, Shimadzu) application for 30 mA or 40 kV was collected between 10°-80° of the XRD measuring range. FESEM showed the diagnostic morphology and the size of the particle. FESEM (Tescan Mira3 FESEM, Czech Republic) in Iran-Mashhad was examined for the specimens. The absorption strength, the vibration mode, and function groups were investigated by FTIR spectra (Spectrum GX FT-IR, Perkin-Elmer).
Mechanism Interaction of Synthesis and Characterization of IONPs from Aloe Vera Extract by Hydrothermal Method as Determinants of Biologic Activity
FeO NPs have unique features due to their tiny size and correspondingly large specific surface area, such as their suitability as catalysts for chemical processes. Given that surface atoms or molecules dominate in defining bulk characteristics and that the proportion of surface to total atoms or molecules rises exponentially with decreasing particle size, the significance of surface area becomes clear. If NPs are ingested into live creatures, assuming they are solid rather than soluble particles, increased surface reactivity predicts that they will demonstrate more biologic activity per given mass than bigger particles (Amato, 1989). In addition, the analysis of the aloe vera extract revealed that it included various vitamins, enzymes, minerals, sugars, lignin, saponins, salicylic acids, and amino acids. Vitamins: It has antioxidant vitamins A (beta-carotene), C, and E. Additionally, it has vitamin B12, folic acid, and choline, which are crucial parts of henna extract. It also studies the breakdown of MB dye for environmental remediation using a hydrothermal approach. Due to the presence of polyphenols, antioxidants, and flavonoids, henna extract helps to transform iron rust into iron oxide nanoparticles (NPs); however, the precise process is still being studied (Abid & Kadhim, 2020). Equation (1) describes the chemical reaction of a mixture to produce FeO NPs. Figure 4 shows the mechanism-interaction of the synthesis and Characterization of IONPs from aloe vera extract by the hydrothermal method as determinants of biologic activity.Fe rest+ Plant extract (aloe veraFeO + HO FeO NPs (1)
The photocatalytic activity for degradation of MB dye using IONPs (Fe3O4 and Fe902O
In this study, IONPs are produced for the photo-catalytic action to remove MB coloring, resulting in a mixture of aloe vera extract and rust iron by the hydrothermal method at (300 and 600) oC. IONPs (Fe3O4 and Fe902O) were used to remove MB dye under average light irradiation, and the process was done by mixing a specific amount of MB (0.001 g) with 100 ml of distilled water. After that, 0.003 g of IONPs powder was scattered, and the solution was stirred by a hotplate stirrer for 10 minutes without light to preserve the IONPs solution's evenness. After 15 minutes, the mixture was put under direct, normal room light. Lastly, about 4 ml of solution was centrifuged at 10,000 rpm for 15 minutes. The specimen's absorbance was measured via the UV-Vis spectrum (Shimadzu, UV-1800), with the absorption maximum at λ = 664 nm. The following formula was used to determine the removal efficiency percentage of MB dye:
The efficiency of the formula (2) as used to remove MB dye is as follows:
Equation (3) was used to get the kinetic constant rate (Kph) of the degradation of the MB dye:
Equation (4) was used to determine the percentage of MB dye degradation that involves IONPs:
Where t is the exposure duration (in minutes), Kph is the kinetic rate constant (in minutes-1), and Cini and Cfin are the beginning and final concentrations of the MB dye, respectively.
Results and Discussion
Synthesis and characterization of IONPs from the mixing of aloe vera with rust iron
It controlled the IONP creation rate, its fill, and its stability by adjusting the parameters of the aloe vera extract. Within an aloe vera extract, phytochemicals have the capacity to produce fewer iron ions in the short term. IONPs prepared from aloe vera minerals also play a crucial role in reducing and stabilizing these causes. Aloe vera extract comprises a range of anthraquinones, glucosidane, parabaloin, and an ingredient. It includes resin, organic and poly acids, aloe-emodins, and a few minerals. A gel in the aloe plant has been found in the leaves, and it has been demonstrated that this gel comprises 0.542% solids, 42% carbohydrates, 1.95% nitrogen, and 0.113% fatty compounds responsible for the production of IONPs.
Color Change
Through the visible color change, it can get first-hand information regarding the formation of IONPs. As the IONPs are created, the color of the solution changes from brown-red to black, which refers to the presence of IONPs. The various colors were due to the change in surface plasmon resonance of IONPs during their formation. The presence of the sources is due to the novelty of the work.
Weight Change
During the experiment, it was observed a loss in the weight of the rust iron, and this was done by measuring the weight of the piece of rust iron before use, and it was 148 g, and after its use, it became 147.01 g, and this is an indication of the disposal of rust iron and the formation of iron extract and their use in the preparation of IONPs. The presence of the sources is due to the novelty of the work.
Figure 5
XRD analysis shows that the crystalline A) Fe3O4 (magnetites,space group of Fd-3m, JCPDS No. 01-089-0951) phase created by mixing the rustiron extract with aloe vera extract was produced at 300 °C, and (B) Fe902Ophase (wustite, space group of FM-3 m, JCPDS No. 01-079-1971) was produced at 600 °C.
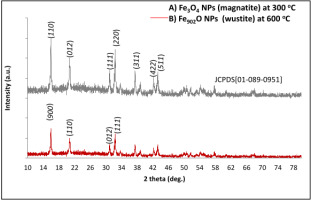
XRD analysis of (Fe3O4 and Fe902O) produced from mixing the rust iron extract with aloe vera
XRD analysis is an excellent instrument utilized to determine samples in this research regarding material, structure, and orientation. IONPs were prepared by mixing the aloe vera with iron rust extract by the hydrothermal method at (300 and 600) oC, as shown in figures 5 (A–B). The high peaks of the crystalline Fe3O4 phase at (110) and (311) are indicated by (110), (012), (111), (220), (311), (422), and (511) of the miller (structure) indexes, as indicated by figure 5 (A) (Ramegowda & Senthil-Kumar, 2015; Sim, Yim, & Choo, 2020). While the high peaks of the crystalline phase Fe902O are (006), (110), (012), and (111), miller indices with the face center cubic, as illustrated in figure 5 (B) (Maham, Nasrollahzadeh, Sajadi, & Nekoei, 2017; Popescu et al., 2020), the peaks of the crystalline phase Fe902O are (006) and (111). Table (1) provides the results in phases and crystalline IONPs (Fe3O4 and Fe902O). The crystallite size D (nm) has been established by the following Scherrer formula 5 (Farshchi, Azizi, Jaafari, Nemati, & Fotovat, 2018; Pawar et al., 2017):
D (nm) =
When k is called a form factor, α is an angle of diffraction, and β is the total width of half (FWHM) (Cittrarasu et al., 2019); λ is the width of wavelength (0.15418) nm (CuKα). The XRD analytical data shows the stages (magnetic, Fd-3m, JCPDS 01-089-0951), prepared from mixing the rust iron extract with aloe vera at 300 °C, in an eco-friendly format and with the best possible orientation, compared with the crystalline Fe902O (Wustite, Fm-3m space groups, JCPDS no. 01-079-1971) prepared from mixing the rust iron extract with the aloe vera at 600 °C.
Table 1
XRD analysis shows the crystallite size forIONPs created from mixing the rust iron extract with aloe vera extract.
Plant extract | Materials | Temperature (oC) | (hkl) | FWHM | Crystallite size D (nm) |
Aloe vera | Fe3O4 (magnatite) | 300 | (110) | 0.43 | 19 |
(220) | 0.30 | 26 | |||
Fe902O (wustite) | 600 | (006) | 0.28 | 28 | |
(111) | 0.13 | 61 |
FE-SEM Images of IONPs (Fe3O4 and Fe902O) from mixing the aloe vera extract with the rust iron
FE-SEM images illustrate the particle size and morphology of the surface area of environmentally friendly IONPs produced by mixing the aloe vera extract at (300 and 600) oC. The average grain volume is 26.80 to 37.96 nm, and the shape of the Fe3O4 NPs (magnetites) is 300 °C (nanosphere), as illustrated in figure 6 (A-B). The particle size and morphology of the Fe902O phase (wustite) are averaged (31.63 to 130.3), as is the (nano-inverse cubic) structure (as illustrated in figure 6 (A1-B1).
Figure 6
FE-SEM images illustrate the outcomes of IONPs generated from the aloe veraextract: A-B) Fe3O4 (magnetic) at 300°C, and A1-B1) Fe902ONPs (wustite) at 600 oC.

FE-SEM images shows the particle size, and the morphology of environmentally compatible (Fe3O4) NPs (magnetites) made from mixing the rust iron extract with the aloe vera extract at 300 oC is better than those prepared for (Fe902O) NPs (wustites) at 600 oC.K
FT-IR spectra of (Fe3O4 and Fe902O) produced from mixing rust iron with aloe vera
The FT-IR spectrum reveals the vibration and function groups of eco-friendly IONPs generated by the hydrothermal method from the rust iron extract with aloe vera at (300 and 600) oC. The absorption strength of the Fe3O4 NPs (magnetite, fingerprint area) is (660) cm-1 at 300 oC, as illustrated in figure 7 (A). In comparison, the absorption band is (650) cm-1 at 600 °C (wustite, fingerprint area), equivalent to Fe-O in Fe902O NPs, as shown in figure 7 (B).
Figure 7
The FT-IR spectrum shows of the IONP preparation from mixing therust iron extract with aloe vera, A) Fe3O4 NPs (magnetite) at 300 oC, and B) Fe902O NP (Wustite) at 600 oC.
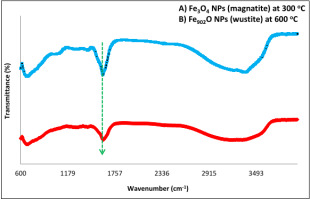
Table 2
The FTIR spectrum shows the values of IONPs from thealoe vera extract with the rust iron.
The FT-IR spectra show the absorption band of eco-conservative (Fe3O4) NPs (magnetites) produced by the aloe vera extract with the rust iron is increased by the hydrothermal method at 300 oC, then Fe902O at 600 oC.
Figure 8
A) UV-Vis spectrum for MB dye removal down room light irradiation using Fe3O4 NPs (magnetite) at 300 oC,and B) Fe902O NPs (wustite) at 600 oC.
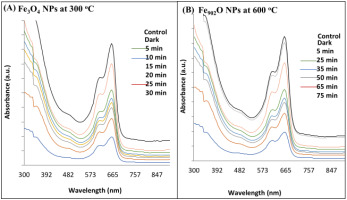
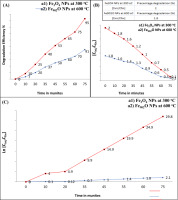
Figure 9
The efficiency of degradation (%) of MB dye is shownusing IONPs prepared by mixing the rust iron extract with aloe vera:(a1) Fe3O4 NPs at 300 oC and (a2) Fe902ONPs at 600 oC, for the room condition. B) a pseudo kinetic curve forthe reaction of the first reaction for adsorption of MB applied (a1) Fe3O4NP at 300 °C, and (a2) Fe902O NP at 600 °C.
The photocatalytic activity for removal of MB dye using IONPs (Fe3O4 and Fe902O)
The application of IONPs prepared from mixing the aloe vera extract with rust iron extract for the MB dye removal. Figure 8 (A–B) showed the removal of MB dye without and using the (Fe3O4 and Fe902O) NPs. The determination of MB dye removal using the (Fe3O4 and Fe902O) NPs when exposed to natural light at room temperature is clean in figures 8 (A–B). When offering the dye to natural light irradiation, it notices that there is no change in the color and concentration of the dye, and they are still within the average extent. However, when the dye is mixed with IONPs under average light irradiation, it is observed that the dye's concentration gradually decreases until it is completely removed at the end (Kim, Lee, Lafferty, Giammar, & Fortner, 2018; Singh et al., 2019; Ullrich, Rahman, Longo, & Horn, 2019; Vaidyanathan, Sendhilnathan, & Arulmurugan, 2007). The reaction mechanism to remove the MB dye first occurs when Fe902O NPs with dye are exposed to natural light at normal conditions. An electron pair will generate a gap after the electrons are blustery from pupil conduction, and then holes are formed in the valence beam. Secondly, the holes form and the electrons interact with water and oxygen in the solution to produce the hydroxyl group, which is characterized by being highly oxidative. Finally, the resulting hydroxyl group obliterates the dye by extracting electrons from the dye molecules via breaking great organic matter into small, less harmful organic matter; this result is similar to the previous study (Dauthal & Mukhopadhyay, 2015; Devi, Begum, & Ahmaruzzaman, 2016; Khodadadi, 2017; Lassoued, Lassoued, Dkhil, Gadri, & Ammar, 2019; Litvin & Minaev, ; Singh et al., 2019; Soltan, Lasssoued, Ammar, & Toupance, 2017; Yuan, 2018). Figure 8 (A-B) shows the removal efficiency (%) for MB dye-down natural light at the normal condition of IONPs created using aloe vera extract at (300 and 600) oC. The degradation efficiency (%) is (95 %) for 40 min of Fe3O4 NPs (magnetite) at 300 oC, but the degradation efficiency (%) was (91 %) for 75 min of Fe902O NPs at 600 oC, as shown in figure (A) (Zhang, Du, Li, Zhang, & Dionysiou, 2011). The percentage of degradation MB dye that is degrading Fe3O4 NPs synthesized using aloe vera extract at 300 oC deposition on glass substrate by drop-casting method is higher than the percentage of degradation MB dye that is degrading Fe902O NPs using aloe vera extract at 600 oC, as explained in figure 9 (B) (Wilhelm, Goss, & Garab, 2020). The constant rate Kph of MB dye of Fe3O4 NPs synthesized using the aloe vera extract at 300 oC deposition on glass substrate by the drop-casting method was determined to be 0.099 min-1, but the constant rate Kph of MB dye of Fe902O NPs was 0.029 min-1, as shown in figure 9 (C) (Ahmad et al., 2015; Brown, 1987; Ibrahim, Al-Razak, Ibrahim, & H, 2013; Oelmüller, 2018; Reaad, Jasim, Aboud, & Hussain, 2023; Xu et al., 2012). IONPs are covered by the numerous phytochemicals found in plant material. The ideal method is to use phytochemicals rather than other chemicals. These are simple to make and don't need to undergo high-temperature aloe vera material degradation in order to be used for this technique. Excellent dye degradation activity may be seen in them. These nanoparticles can be reused or recycled. When we add leaf extract from the aloe vera plant to the iron rust solution during the synthesis of the IONPs, the solution changes color and produces the IONPs. The IO surface was excited, which led to the creation of color. For the oxidation of the dye MB, highly active plant-assisted Fe902O NPs exhibit enhanced catalytic activity (Khalel, Hamza, & Khaled, 2023).
Conclusion
This work has succeeded in producing IONPs (Fe3O4 and Fe902O) by the hydrothermal method, utilizing modern methods of mixing plant extracts such as aloe vera and rust iron extract. XRD measurements explained the small size of the crystalline (19 nm) magnetite structure of (Fe3O4) NPs at 600 oC using aloe vera extract, while the tiny size of the crystalline (Fe902O) NPs at 300 oC was at 28 nm. FE-SEM show the particle size of (Fe3O4) NPs at 600 °C for (26.80 to 37.96) nm, while the tiny crystalline size of the Fe902O NPs (wustite) at 300 °C was (31.63 to 130.3) nm. The FT-IR spectrum explains the strong absorption band of Fe3O4 NPs (magnetites) at 600 °C. The FT-IR spectra absorption band was (650) cm-1, and were corresponding to Fe-O at 300 °C (Fe902O NP). In a relatively short time, IONPs for the breakdown of MB dye have been used in environmental treatment. The efficiency of degradation (percentage) for MB dye was 95% (40 minutes) for IONPs (Fe3O4) at 300 °C. In comparison, the efficiency of degradation (percent) for MB dye was 75 minutes (91%) with (Fe902O) NPs at 600 °C. This work will provide us with wide-ranging new horizons to solve the problem of rust iron and how to remove it and produce new NPs when it comes to the environmental treatment of removing poisonous colors from water at accessible prices.