Introduction
Malvaceae Juss. family has a global distribution, especially in tropical and temperate regions, composed by almost 250 botanical genera (APG IV, 2017). Particularly, the neotropical genus Ceiba Mill. has 18 species that occur mainly in seasonally dry tropical forests (SDTFs) and tropical forests. The largest trees, about 50 m tall, are present in seasonally flooded várzea forests and the smallest, reaching 2 m, in rocky outcrops (Pezzini et al., 2021).
Ceiba are morphologically characterized by the presence of robust and aculeate trunks, leaves alternate compound-digitate, 5-7 leaflets with serrate margins, solitary flowers or in inflorescences, these being pentamerous and dichlamydeous, and fused staminal filaments that form a staminal tube (Figueiredo, Monteiro, Da, & Melo, 2020).
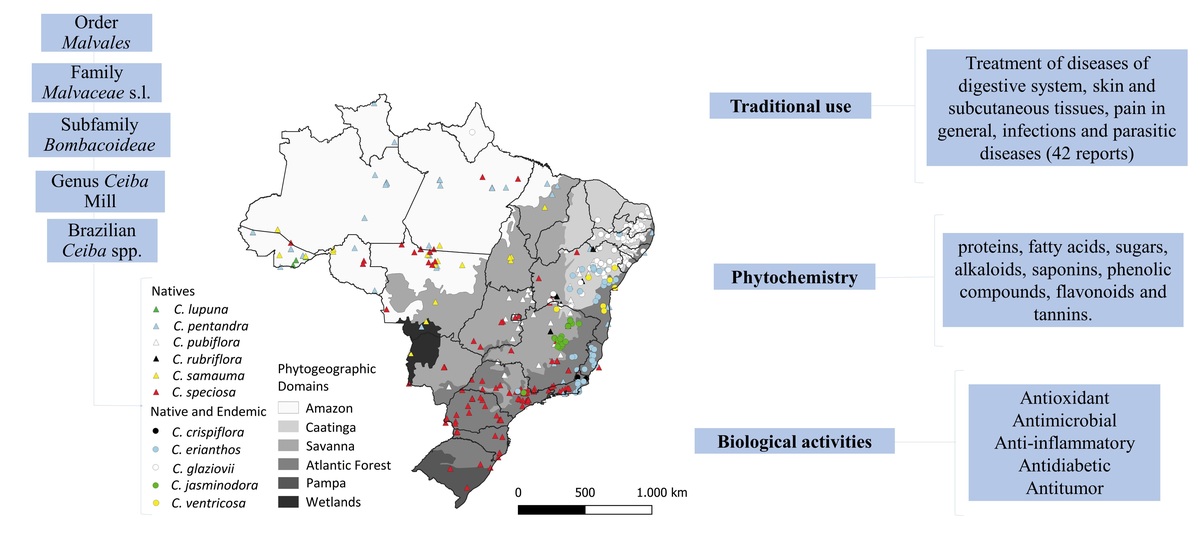
In Brazil, these species are distributed on all Brazilian geographic regions (North, South, Midwest, Northeast and Southeast) and in its six phytogeographic domains (Amazon, Caatinga, Cerrado, Atlantic Forest, Pampa and Wetlands). There are 11 Brazilian native Ceiba species, six of which are also considered endemic, with highest prevalence in country´s northeast region (REFLORA, 2022).
These genus species are used in landscaping and urbanism due to their large branches and brightly colored flowers, in addition to their economic and commercial applications, such as wood and vegetable oils and fibers production (as from C. speciosa and C. pentandra fruits) (Tripathi, Farooqui, Singh, Singh, & Roy, 2019). Medicinal application of these species is also observed, in traditional medicine it is used for treating diabetes (Júnior, Andrade, Araújo, Barbosa, & Barbosa, 2014), high blood pressure (Albuquerque et al., 2007; Almeida, Silva, Amorim, Maia, & Albuquerque, 2005), inflammations (Sobrinho et al., 2021), chest and spine pains (Ribeiro et al., 2014; Silva, Marinho, Lucena, & Costa, 2015), diarrhea (Ndenecho, 2009; Nwachukwu, Allison, Chinakwe, & Nwadiaro, 2008), and rheumatism (Agra, Baracho, Nurit, Basílio, & Coelho, 2007; Agra, Silva, Basílio, Freitas, & Barbosa-Filho, 2008; Sanz-Biset, Campos-de-la-Cruz, Epiquién-Rivera, & Cañigueral, 2009). Consequently, the number of scientific studies that seek to validate such knowledge has been increased in the last decade.
There are several literature reports about these species bioactive potential, examples are C. pentandra, as antidiabetic (Fofie et al., 2019), antimicrobial (Santos, Jacinto, & Cruz, 2021), antiviral (Dewi et al., 2019), and antitumor (Abouelela, Orabi, Abdelhamid, Abdelkader, & Darwish, 2018). Another that is frequently cited is C. speciosa, for its antidiabetic (Santos et al., 2020) and antioxidant properties (Braga et al., 2019).
Although there are a relevant number of publications about Ceiba spp., their restricted geographic distribution, reduce the scientific studies interest. Moreover, the absence of ethnopharmacological and/or ethnobotanical surveys restrains their information, especially for Brazilian endemic species. Thus, this review aims to compilate the available information about Ceiba species to offer a scientific literature update and to help on understanding its ethnopharmacology, chemical composition, and biological activities in an integrated manner.
Materials and Methods
For this integrative review design, Flora e Funga do Brasil database was used, due to its attribution on recording, grouping, and disseminating data regarding Brazil plant specimen’s occurrence, and 11 Ceiba Mill. species were reported on this database in Brazil at the search period (http://reflora.jbrj.gov.br). Then, the review based on collection of evidences, especially for those with chemical, bioactivities, or ethnobotanical approaches.
Literature review was carried out between June 2020 and January 2023, through selecting articles in English, Portuguese, Spanish and French, indexed in databases ScienceDirect, PubMed, and Google Schoolar, with the main keywords: Ceiba crispiflora, Ceiba erianthos, Ceiba glaziovii, Ceiba jasminodora, Ceiba lupuna, Ceiba pentandra, Ceiba pubiflora, Ceiba rubriflora, Ceiba samauma, Ceiba speciosa and Ceiba ventricosa, in addition to the synonyms Chorisia crispiflora, Chorisia glaziovii, Chorisia pubiflora, Chorisia speciosa and Chorisia ventricosa. In addition, the keywords ethnopharmacology, medicinal plants, biological activities, pharmacological potential were also used. The selection criteria were based on the presence of reports about botanical, phytochemical, or pharmacological activities of these 11 species. Studies regarding other Ceiba species, and non-pharmacological activities were removed.
Morphology and distribution in Brazil
Ceiba Mill. taxonomic history presents divergences, because of species that currently integrate it have already composed other genera, such as Chorisia (APG IV, 2017). Morphological characteristics that differentiated the two genera are the lower staminal filaments arrangement in a corona-like structure, and the upper filaments fused to form the upper staminal tube. This last structure is absent in Ceiba, which has only the lower staminal tube, which divides into five free stamens at the top (Gibbs & Semir, 2003). However, after morphological analysis of pollen grains, a union between the two genera was proposed, and the classification of Ceiba is currently used for both genus (Gibbs, Semir, & Cruz, 1988).
Ceiba spp. trees most often have a height between 15 to 20 m, however C. pentandra can reach more than 50 m, being the tallest member of the group. Trunks are straight, robust, and aculeate, and, in some species, such as C. speciosa, C. glaziovii and C. pubiflora, may have ventricose or “swollen” trunks, presenting a more robust area in stem middle portion. This particularity in its structure explains some common names given to these trees in South America, such as “barriguda” (in literal translation would be “paunchy”), in Brazil, and “palo borracho” (in literal translation would be “drunken tree”) in Peru, Bolivia and Argentina (Gómez-Maqueo & Gamboa-Debuen, 2022; Pezzini et al., 2021). Trunk also culminates in a dense canopy, with alternate compound-digitated leaves, long petioles, and leaflets (in number of 5 to 7) with serrated margins. Flowers can be solitary or organized in fasciculate inflorescences of few flowers, presenting deciduous bracts (Gibbs & Semir, 2003).
Fruits have an ellipsoidal capsule shape and endocarp develops into a fine white cotton fiber (named kapok), which is why they are known as “silk cotton tree” and “kapok tree”. This fiber is capable of protecting the seeds and assist in wind dispersion. This genus representatives show great morphological similarity but can be distinguished by differences in staminal filaments, in which these can be fused, forming a tube around the ovary (called staminal tube). Staminal filaments also can divide into five free filaments in some species, or remain fused just above the stem appendages, forming the upper staminal tube (Gibbs & Semir, 2003).
Among the cataloged species, eleven are found in Brazil and six of them are native. The ones found in Brazil are: Ceiba lupuna P.E.Gibbs & Semir, Ceiba pentandra (L.) Gaertn., Ceiba pubiflora (A.St.-Hil.) K.Schum., Ceiba rubriflora Carv.-Sobr. & L.P.Queiroz, Ceiba samauma (Mart.) K. Schum. and Ceiba speciosa (A.St.-Hil.) Ravenna, and five others species are native and endemic to the Brazilian territory: Ceiba glaziovii (Kuntze) K. Schum., Ceiba crispiflora (Kunth) Ravenna, Ceiba erianthos (Cav.) K .Schum., Ceiba jasmindora (A.St.-Hil.) K. Schum. and Ceiba ventricosa (Nees & Mart.) Ravenna (REFLORA, 2022).
These species are distributed on all Brazilian geographic regions (North, South, Midwest, Northeast, and Southeast) and in its six phytogeographic domains (Amazon, Caatinga, Cerrado, Atlantic Forest, Pampa, and Pantanal), as can be seen in Figure 1 (SiBBr, 2022). They occur, mainly, in rocky outcrops, rocky fields, limestone fields and seasonally dry tropical forests (STDFs) (Figueiredo et al., 2020; REFLORA, 2022). Although most of the species are present in STDFs, C. jasminodora is found only in rocky outcrops, and other species are also present in humid habitats, such as C. speciosa and C. samauma, present in several South American countries. C. pentandra has a pantropical distribution and C. lupuna is restricted to rainforests (Gibbs & Semir, 2003; Pezzini et al., 2021).
Data for this map constrution was obtained from Instituto Brasileiro de Geografia e Estatística (IBGE), Interface Integrada para Internet de Ferramentas de Geoprocessamento (i3GEO) created by Ministério do Meio Ambiente (MMA), and Sistema de Informação sobre a Biodiversidade Brasileira (SiBBr). Map was constructed using QGIS software.
Medicinal tradicional use
Medicinal knowledge is part of the characteristic habits and customs of traditional communities and is used for the prevention and treatment of diseases. Traditional medicinal knowledge is transmitted orally or through written narratives, being fundamental in maintaining the physical, emotional, and spiritual integrity of the community (García-Flores, González-Espinosa, Lindig-Cisneros, & Casas, 2019).
This knowledge is extremely relevant, about 80 % of the world population uses herbal medicines and, in tropical countries, two thirds of the plant species used are obtained directly from nature (Albuquerque et al., 2011). In traditional rural communities, these practices are even more evident, as access to health services can be restricted (Saraiva et al., 2015).
From the traditional knowledge to prospecting new drugs, the first one cannot be totally discarded since it helps in the search for compounds with the most diverse bioactivities, which has been a successful tool for new medicinal alternatives development over the last few decades (Ribeiro, Bieski, Balogun, & Martins, 2017; Saraiva et al., 2015).
Several ethnopharmacological uses are reported for Ceiba species in Brazil and in other tropical countries of the world, as can be seen in Table 2. 42 reports were found, among them, C. pentandra was the most cited (25) followed by C. glaziovii (11), C. speciosa (3) and C. pubiflora, C. samauma and C. ventricosa, with only 1 report each. C. crispiflora, C. erianthos, C. jasminodora, C. lupuna and C. rubriflora were not mentioned in the consulted literature as used for traditional medicinal purposes.
Interestingly, C. glaziovii is found exclusively in Caatinga phytogeographic domain, in Brazil (Gibbs & Semir, 2003), and although it has a restricted distribution, it presents a significant number of ethnobotanical studies, which places this species in prominence. Although there is considerable literature on its ethnopharmacological uses, there are no experimental studies that confirm the medicinal properties of this species (Saraiva et al., 2015).
Modes of preparation
The use of various plant elements was found in the consulted literature, totaling 57 reports contained in 42 studies. The bark/stem (bark, inner bark, inner bark, stem and trunk) was the most cited element (30), followed by leaves (8), roots (7), flowers (6), fruits (3), xylem/heartwood (2) and resin (1). The higher recurrence of the popular use of elements related to the bark/stem can be observed in other ethnobotanical approaches. This may be related to the perennially of these elements in the plant during seasonal changes, easy collection and storage, higher concentration of bioactive substances, and preservation of therapeutic properties for long periods of time (Júnior et al., 2014; Saraiva et al., 2015; Sobrinho et al., 2021).
Regarding the methods of medicinal plants preparations, 42 citations were found in the 31 articles consulted, distributed in five different ways: decoction, infusion, maceration, syrup, and percolation. Decoction and infusion have the highest number of reports, with 19 and 6 citations, respectively. They consist of the extraction of water-soluble constituents; in the decoction, the vegetable raw material is boiled in an open extractor to obtain thermostable compounds, and in the infusion, the vegetable material macerated in cold or boiling water is used to obtain the phytochemicals (Manousi, Sarakatsianos, & Samanidou, 2019).
The least mentioned methods of preparation were maceration, syrup, and percolation, with 3, 2 and 1 citations, respectively. In maceration, the material is ground and placed in a container, covered by the solvent, and kept for, at least, three days. Percolation is a more sophisticated technique that uses a dedicated device, in which the plant raw material is placed inside it as an immobile bed, and extraction occurs by passing the solvent in a continuous flow (Abubakar & Haque, 2020). Syrups are prepared with sugar or honey and are generally used to treat diseases in children, being popularly known as “lambedor” in Brazil (Agra et al., 2008).
Treated diseases and administrations forms
In the consulted literature, there are 17 reports of forms of medicinal administration of Ceiba species. The most cited is the oral intake (12 reports), with the use of teas, juices, and syrups, followed by topical use (3 reports), washing the affected areas (2), and mouthwash and contact with the cervix, with 1 citation each.
Several therapeutic indications are cited for the medicinal use of Ceiba species (64 occurrences were reported) in 42 articles, categorized according to the classification proposed by Albuquerque et al. (2007) with modifications. Use indications for treatment Diseases of the digestive system and Diseases of the skin and subcutaneous tissues are the most cited categories (8 reports each), as can be seen in Table 1. Followed by afflictions and pains not defined (7), Infectious and parasitic diseases and Injury, poisoning and other infirmities with external causes (6 each), Diseases of the respiratory system and endocrine, nutritional and metabolic diseases (5 each), Diseases of the musculoskeletal system and connective tissues and Pregnancy, childbirth and the puerperium (4 each), Diseases of the genitourinary system, Diseases of the circulatory system, and Diseases of the blood and blood-forming organs (3 each), and Neoplasias (2).
Table 1
Number of medicinal indications for Ceiba species in traditional use, grouped into categories.
Phytochemical composition
Plants produce several chemical constituents that play critical roles in these sessile and multicellular organisms, making them capable of inhabiting various environmental niches. These compounds can be from primary metabolism, such as amino acids, carbohydrates, nucleotides, and fatty acids, or from secondary metabolism (also called specialized), as phenols, terpenes, phenolic acids, and alkaloids (Maeda, 2019) .
Secondary metabolites are essential for interaction with the environment, although their presence may diverge for each species. Biosynthesis of secondary metabolites varies according to the environmental conditions on the plant imposed, such as climatic aspects, seasonality, temperature, and humidity, generating specific responses on the phenology (Isah, 2019). Ceiba spp. native of Brazil have a great diversity of primary and secondary metabolites, which are obtained from different parts of these plants.
Table 2
Uses of Ceiba genus species in traditional medicine.
Ceibapentandra
C.pentandra has a vast scientific literature about its chemical composition around the world. Stem bark has phenols (Nwachukwu et al., 2008), tannins, catechins, mucilage (Kone et al., 2012), C-glycosides, reducing sugars, triterpenes (Bairwa, Sethiya, & Mishra, 2010), tannins, flavonoids, alkaloids, terpenoids, saponins (Syihabudin, Sari, Utami, & Apriliani, 2018), resins, proteins and steroids (Akaneme, 2008). Aerial parts have saponins, tannins, alkaloids, essential oils (Kubmarawa et al., 2007), hydrocarbons and fatty acids (Abouelela et al., 2018), and the leaves of C. pentandra have carotenes (Smith, Dueker, Clifford, & Grivetti, 1996), vitamins, proteins (Dickson et al., 2012), fatty acids, mono and polysaccharides, fiber (Herzog, Farah, & Amadò, 1993), alkaloids, phenols (Bhavani, Bhuvaneswari, & Rajeshkumar, 2016), saponins, flavonoids, terpenoids and steroids (Bhuvaneswari, Balaji, Prakash, & Kannaian, 2014).
Seeds were also investigated for their composition, having phenolic compounds, flavonoids, alkaloids, tannins (Kiran, Rao, Sirisha, & Rao, 2015), fatty acids, proteins, fibers and phytosterols (Anwar, Rashid, Shahid, & Nadeem, 2014). Essential oil obtained from stem bark present as major constituents β-caryophyllene (28.7%), β-elemene (18.5%), α-muurolene (7.8%), caryophyllene oxide (4.8%) and α-humulene (4.2%), and heartwood essential oil have α-eudesmol (21.1%), 2-ethoxyacetate (11.3%) and nonanal (7.3%) as main compounds (Alade et al., 2021). In addition to these compounds mentioned above, literature also report a great diversity of isolated phytocompounds from this species, as can be seen in Table 3. Despite the high number of studies that use C. pentandra as an object of investigation, no research produced in Brazil was found, even though this species has a wide distribution in the Brazilian territory (Figure 1).
Ceibaspeciosa
Phytochemical analyzes of C. speciosa are reported around the world, using several parts of the plant, as bark, seeds, leaves, and fruits. Branches aqueous extract has a wide variety of phenols and flavonoids, such as quercetin, rutin, and gallic, caffeic and chlorogenic acids, and a total dosage of phenolic compounds of 117.4 ± 6.2 mg GAE/g (milligrams equivalent of gallic acid per gram of extract) (Dörr et al., 2019). In dosages to determine the quantity of flavonoids and polyphenolic compounds from trunk aqueous extract, values of 240 μg of quercetin/g and 425 μg of gallic acid/g were found, respectively (Malheiros et al., 2017). Also, in this study, the phytochemical profile of C. speciosa bark ethanolic extract was evaluated, with total flavonoids dosage (429 μg of quercetin/g), total polyphenols (470 μg of gallic acid/g), and identification by high performance liquid chromatography (HPLC) of various phenolic compounds, such as kaempferol, quercetin, rutin, caffeic, chlorogenic, gallic, and ellagic acids.
Leaves aqueous extract also has a low content of phenols (3.3 mg gallic acid/g extract) and flavonoids (7.7 mg quercetin/g extract) (Krishnaveni, Amsavalli, Chandrasekar, Madhaiyan, & Durairaj, 2013), and leaves ethanolic extract has triterpenes (β-amyrin), sterols (stigmasterol), organic acids (p-hydroxybenzoic and succinic acids) and glycosides (verbascoside, β-sitosterol-3-O-β-d-glucopyranoside, astragalin, cinnaroside, tylyroside and rhiofolin) (Nasr, Assaf, Darwish, & Ramadan, 2018).
Fruits, more specifically mesocarp and the exudate gum, have several carbohydrates, such as rhamnose, arabinose, xylose, mannose, glucose, and galactose (Beleski-Carneiro, Ganter, & Reicher, 1999). Seed oil, submitted to gas chromatography followed by mass spectroscopy (GC-MS), presented a rich lipid profile, being found as major constituents: linoleic (28.22 %), palmitic (19.56 %), malvalic (16.15 %), sterculic (11.11 %), and dihydrosterculic acids (2.74 %) (Rosselli et al., 2020).
C. speciosa is also capable of producing essential oils, which, after being obtained from the leaves, were identified through GC-MS as major components: caryophyllene (32.26 %), bicyclogermacrene (9.88 %), humulene (9.26%), α- selinene (9.18 %), and β-elemene (8.06 %) (Kausar et al., 2020).
Other species
Other species of Ceiba genus that are native to Brazil had qualitative and quantitative analysis of its phytocompounds. In studies with the ethanolic extract of C. glaziovii bark, the presence of phenols, tannins, triterpenes and quinones was identified (Almeida et al., 2005). In bark and leaf hydroalcoholic extracts, catechin tannins, flavonols, phenols, flavones, xanthones, alkaloids, anthocyanins, anthocyanidins, saponins and proteins were found (Leal, Dantas, Chaves, Felismino, & Vieira, 2011). In studies with Ceiba pubiflora flowers hydroethanolic extract, a quantitative analysis of total phenols was performed, obtaining 7.26 ± 0.16 mg GAE/100g. In qualitative analysis, presence of chemical groups such a cyanogenic glycoside, reducing and non-reducing sugars, tannins, flavonoids, alkaloids, coumarins, saponins, organic acids and steroids were identified (Menezes-Filho, Santos, Castro, Oliveira-Filho, & Christofoli, 2022).
Biological activities
Ceibapentandra
The main biological activity cited for C. pentandra is antidiabetic. Studies report its ability to inhibit carbohydrate digestive enzymes, a property that can help control blood glucose of patients, especially in reducing postprandial hyperglycemia. Stem bark methanolic and aqueous extracts showed inhibition of α-amylase with IC50 values of 6.15 and 54.52 μg/ml, and α-glucosidase of 76.61 and 86.49 μg/ml, respectively (Nguelefack, Fofie, Nguelefack-Mbuyo, & Wuyt, 2020). Leaves methanolic extract showed weak inhibition of α-amylase (5.86 %), and high total content of phenolic compounds and flavonoids, in addition to potent free radical scavenging, and efficient reducing power (RP) as compared with other plant in this study, showing a low correlation between antioxidant activity and enzymatic inhibition (Oyedemi et al., 2017). Bark hydroalcoholic extract fractions show promising α-glucosidase inhibition activity, in which IC50 values of 4.60, 8.55 and 5.61 μg/mL for n-hexane, ethyl acetate and aqueous fractions, respectively, having approximate values to quercetin (6.04 μg/mL), a flavonoid used as a standard (Syihabudin et al., 2018).
Table 3
Isolated compounds from C. pentandra.
In vitro antihyperglycemic activity of bark decoction was also observed, in hyperglycemic milieu, there was a significant increase in glucose uptake by the liver (56.57 %) and skeletal muscle (94.19 %), and in hypoglycemic milieu, there was a reduction in glucose release by the liver (33.94 %) (Fofie et al., 2014). In in vivo evaluations, trunk bark aqueous and methanolic extracts of C. pentandra can improve peripheral glucose uptake, insulin resistance, lipid metabolism, and oxidative state. The methanolic extract (200 mg/Kg) caused a glycemic reduction of 62.4%, when administered to diabetic rats, in addition to improving oral glucose tolerance, and physiological changes caused by diabetes, such as cholesterol and triglycerides, body and pancreas weight, and antioxidant enzymes concentration such as glutathione and malondialdehyde (Fofie et al., 2019).
Bark decoction (150 mg/Kg) can reduce blood glucose by 33 % in oral treatment, and correct impaired glycaemia in rats with insulin resistance induced by dexamethasone, improving dyslipidemia and increasing levels of nitric oxide and antioxidant enzymes such as catalase and glutathione (Fofié, Nguelefack-Mbuyo, Tsabang, Kamanyi, & Nguelefack, 2018). Ethyl acetate fraction of leaves ethanolic extract was able to reduce glycemia in rats with alloxan-induced diabetes, with high reduction observed to the treatment with 200 mg/Kg. In addition to reduction of serum levels of kidney (urea and creatinine) and liver (ALP, ALT and AST) injury markers, compared with untreated group (Lami et al., 2015).
Antifungal and antiviral potential of C. pentandra derivatives were also investigated, as well as the antimicrobial activity. Leaves ethanolic extract acts on virulence factors of Pseudomonas aeruginosa, showing significant inhibition of biofilm formation (1.00 mg/mL) compared to control norfloxacin (2.33 mg/mL) (Santos et al., 2021). Bark hexane and dichloromethane extracts and their fractions show inhibitory activity on the quorum sensing system (a communication system between bacteria) of P. aeruginosa and Chromobacterium violaceum. They also can act on virulence factors such as pyocyanin, alkaline protease and violacein production, being associated with the large quantity of steroids, terpenes, and flavonoids present (Muñoz-Cázares et al., 2018).
Ethanol and aqueous extracts from different parts of C. pentandra show weak activity against fungi that cause dermatitis (Epidermophyton flocosum, Microsporum canis, Trichopyton rubrum and Candida albicans), with values of minimum inibitory concentration (MIC) higher than 50 mg/mL for all microorganisms (Nwachukwu et al., 2008).
Afzal et al. (2022) investigated the antibacterial and antibiofilm activity of silver nanoparticles (AgNPs) synthesized with methanolic extracts from the bark (AgNPb) and leaf (AgNPl) of C. pentandra. Using the plate diffusion methodology, significant antimicrobial activity of AgNPl was observed, with zones of inhibition of 8.6 ± 1.2 mm, 15.0 ± 1.0 mm, and 9.3 ± 2.3 mm against the microorganisms P. aeruginosa, E. coli and S. aureus, respectively, and for AgNPb, with 14.7 ± 1.2 mm, 15.3 ± 0.6 mm, and 12.7 ± 2.3 mm for the same microorganisms. The minimum inhibitory concentration (MIC) for AgNPb was 63 µg/mL against S. aureus, and 125 µg/mL against P. aeruginosa and E. coli, and for AgNPl, it was 125 µg/mL against E. coli and S. aureus and 250 µg/mL against S. aureus and P. aeruginosa for AgNPl. Regarding biofilm formation, AgNPb showed greater inhibition against E. coli, and AgNPl showed moderate activity against the tested microorganisms. The presented antimicrobial potential is attributed to the presence of active biomolecules, such as alkaloids, flavonoids, tannins, and glycosides.
Antiviral action can be observed within its leaves ethanolic extract, which shows expressive inhibition of human liver cells infectivity to dengue virus (DENV), with an IC50 of 15.49 µg/mL, and low half-cytotoxic concentration (CC50 of 81.1 μg/mL) at the lowest concentrations tested, resulting in an infectivity index of 5.23 (Dewi et al., 2019). This antimicrobial and antiviral activity is attributed to the presence of a great diversity of secondary metabolites compounds, especially belonging to flavonoids and tannins classes.
Stem bark ethanolic extract showed strong antitrypanosomal activity against Trypanosoma brucei brucei, with an IC50 of 11.70 μg/mL. Antihemintic activity was also observed, killing Phetima posthuma after the experimental exposure time at 2.5, 5 and 10 mg/mL. These activities may be due to a high concentration and number of tannins and presence of n-hexadecanoic acid, a compound known for its antitrypanosomal potential (Obeng et al., 2022).
Anticancer potential was also observed as in dichloromethane fraction of the aerial parts methanolic extract, which showed promising cytotoxicity against cancer cells, presenting IC50 values of 14,89 and 18,86 μg/mL, against cell lines of hepatocellular carcinoma (HepG2) and breast cancer (MCF-7), respectively. Molecules isolated from this same fraction also had anticancer activity through molecular docking analysis. The compounds 3β-taraxerol, 3β-taraxerol acetate, all-trans-squalene and β-amyrin, have predicted activity for the treatment of diseases proliferative, apoptosis agonists and antineoplastic agents (Abouelela et al., 2018).
Silver nanoparticles synthesized with bark ethanolic extract produce a significant cytotoxic effect against human colon cancer cells (HCT-116) with a IC50 of 60 μg/mL in MTT assay, inducing increased production of reactive oxygen species, which leads to cell membrane damage and subsequently apoptosis (Brian & Selvi, 2019).
Petroleum ether (PE) and acetone (AC) extracts from C. pentandra bark showed robust short-term cytotoxic effects against Ehrlich ascites carcinoma (EAC) cells and long-term cytotoxic effects on human breast cancer cell lines (MCF-7) and melanoma cell lines (B16F10). Mice inoculated by EAC-induced liquid tumor and treated with the extracts had increased survival, and in Dalton’s lymphoma ascites (DLA)-induced solid tumor model, there was a significant reduction in tumor weight and volume (about 50%) compared to negative control. Hematological parameters (WBC, RBC and hemoglobin content) and endogenous antioxidant levels in liver (catalase, SOD, GSH, GST and MDA) were improved, and these may play an important role in antitumor activity of these extracts (Kumar, Kumar, Ramalingayya, Setty, & Pai, 2016).
Seeds fixed oil has in vivo anti-inflammatory potential confirmed, through oral administration in mice, reducing the serum concentration of the inflammatory marker C-reactive protein. It was observed values of 1.08 ± 0.02 and 1.08 ± 0.03 mg/L to 50 and 100 mg/Kg, respectively; similar results to the control sodium diclofenac (1.04 ± 0.04 mg/L). The fixed oil shown blood cells membrane-stabilization, with maximum inhibition 28.8 % at 50 mg/mL. Membrane-stabilizing compounds can act in the initial phase of inflammation, preventing the release of phospholipases that trigger the formation of cytokines. The inhibition of production and function of these inflammatory mediators are fundamental in controlling the inflammatory process (Kiran & Rao, 2014).
Antipyretic activity was observed in leaves ethanolic extract, using murine models, with higher effect at 189 mg/Kg. There was a temperature variation of -0.85 ± 0.58 ºC after 4h of fever induction, compared to the pre-treatment temperature. One of the possible mechanisms for the antipyretic action may be associated with inhibition of prostaglandin E2 (PGE2), responsible for adjusting body thermoregulation in cases of fever (Saptarini & Deswati, 2015). Parhan (2021) evaluated antipyretic potential of leaves ethanolic extract in rats with high body temperature induced by DPT-HB vaccine, finding moderate potential at a concentration of 300 mg/kg of body weight.
Ceibaspeciosa
Recent studies show that C. speciosa has antidiabetic properties, which confirms its ethnopharmacological use, used to reduce cholesterol, triglycerides, and blood glucose levels (Malheiros et al., 2017). Bark aqueous extract can increase glucose utilization and regulate insulin levels. These results were observed in protocols with the nematode Caenorhabditis elegans, with reduced body glucose levels and increased longevity of the animals, protecting them against glucotoxicity (Santos et al., 2020). Seed fixed oil has anti-obesity potential, by slightly inhibiting α-amylase, α-glucosidase, and pancreatic lipase enzymes activity, with IC50 values of 135.69, 158.22 and 127.57 μg/mL, respectively (Rosselli et al., 2020).
Antioxidant capacity of C. speciosa stem bark aqueous extract was evaluated observing high values by the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging methodology (IC50 42.87 μg/mL), moderate effect on ferric reducing antioxidant power assay (FRAP) (236 ± 18 μM FeSO4/g) and slight activity by the oxygen radical absorbent capacity assay (ORAC) (2351 ± 136 μmol Trolox Equivalent/g). These results are attributed to kaempferol presence, a flavonoid known for its antioxidant activity, in addition to other phenolic compounds present in this extract (Dörr et al., 2019).
In another study, the antioxidant activity of bark trunk extracts against the DPPH radical was evaluated, obtaining percentages of 85.13 % and 88.95 % (50 µg/mL) of inhibition, to aqueous and ethanolic extracts, respectively. Other concentrations were also tested (10, 5 and 2 µg/mL) for both extracts and decreasing of activity in dose-dependence was observed (Malheiros et al., 2017). These results differ significantly from those presented by Nasr et al. (2018), that, evaluating C. speciosa bark ethanolic extract, found a lower inhibition percentage, even though concentration tested was higher (1 mg/mL), obtaining 81.2 % of radical scavenging. The specimens collected in the studies come from different geographic locations and climatic conditions. It is expected that there will be variations in the synthesis of secondary metabolites, and therefore in the intensity of the biological activity evaluated.
The antioxidant potential of C. speciosa leaves ethanolic was 78.7 % for DPPH radical scavenging (at 1 mg/mL), with this activity attributed to the presence of phenolic compounds, especially flavonoids (Nasr et al., 2018). Methanolic extract of leaves and its dichloromethane fraction presented antioxidant potential similar to the standard, with IC50 of 15.48 and 12.37 µg/mL, compared to ascorbic acid (7.60 µg/mL) (Abdel-Aziz et al., 2021). Seed fixed oil showed strong IC50 values, with 10.21 μg/mL and 77.44 μg/mL for ABTS and DPPH radical scavenging activity assays, respectively (Rosselli et al., 2020).
Flower pigment of C. speciosa (FPCS) presents strong antioxidant effect, with scavenging the DPPH and ABTS free radical with an IC50 of 49.78 and 58.14 μg/mL, respectively. Pretreatment with FPCS prevents lipopolysaccharide-induced hippocampal oxidative stress in a model using Kunming mice. There was a reduction in the expression of the oxidative stress biomarkers nitric oxide synthase (iNOS) and heat shock protein 60 (HSP60) at concentrations of 30 and 60 mg/kg. There was no acute oral toxicity at the concentrations tested (1 - 10 g/Kg) (Chen et al., 2022).
The antipyretic activity was evaluated in hydroalcoholic extracts of stem and leaves of mice previously inoculated with yeast. A relevant decrease in body temperature was observed in mice previously inoculated with yeast, with maximum effect (400 mg/Kg) reached about the third hour of the experiment, for both extracts. In the evaluation of anti-inflammatory activity by the paw edema methodology, there was a moderate reduction in edema. The most expressive inhibition values were observed after 1 h of test (3-5 h), both for the ethanolic extracts of leaves and stem, and for the isolated compound rhoifolin. This indicates that the extract acts in the second phase of the inflammatory process, and the presence of flavonoids, sterols and triterpenes in these extracts may be associated with inhibition of the release of lysosomal enzymes and prostaglandins (Nasr et al., 2018).
Antiulcerogenic and anti-inflamtory activity of stem bark ethanolic extract of Ceiba speciosa was evaluated, observing the viability of cells of gastric origin (MN01, ACP02 and ACP03), and murine macrophage (RAW264.7) in which ethanolic extract (100µg/mL) showed a proliferative effect. It is also observed the inhibition of the activity of p38α (1.66 µg/mL), JAK3 (5.25 µg/mL), and JNK3 (8.34 µg/mL), and reduction of the release of TNF-α and IL-6 by murine macrophage. In an in vivo evaluation, it reduced the recruitment of leukocytes, significantly reverting the production of NO and reducing the formation of edema in a dose-dependent manner at the concentrations tested (10, 50 and 100mg/Kg), reversing the effects caused by carrageenan. The extract showed significant prevention of ulcer formation at the tested concentrations (20-400mg/Kg) with a greater reduction than the reference drug, Omeprazole (40mg/Kg). This potential is attributed to the phenolic compounds present in the extract, which have antioxidant activity, inhibiting oxidative stress, reducing NO production, and preventing the activation of p38, which, in turn, inhibits TNF-α and IL-6, exhibiting activity anti-inflammatory and anti-ulcerogenic properties (Dörr et al., 2022).
The anticancer activity was evaluated with fractions of the methanolic extract of the leaves of C. speciosa, being observed moderate cytotoxicity against the cell line HepG2. The petroleum ether, ethyl acetate and dichloromethane fractions presented IC50 of 74.35, 79.73 and 57.30 µg/mL, respectively. Dichloromethane fraction still present strong antimicrobial activity, with clear zone of 23, 22, 22 and 21 (
Other species
Hydroalcoholic extract of Ceiba glaziovii, in an antimicrobial activity evaluation against Staphylococcus aureus, showed a not very expressive result in inhibiting bacterial growth, for both bark and leaf extracts (Leal et al., 2011). C. pubiflora leaves hydroethanolic extract has antioxidant potential evaluate by DPPH radical scavenging methodology, with an IC50 of 217.4 ± 0.19 mg/L (Menezes-Filho et al., 2022).
Purified compounds from Ceiba species are also investigated, such as rhoifolin, isolated from C. crispiflora leaves. This compound has neuroprotective properties evaluated in models with zebra fish, being able to improve symptoms of anxiety, memory deficit and brain oxidative stress, inhibiting acetylcholinesterase activity and reestablishing cholinergic activity, thus being a promising compound for amnesia and anxiety treatment (Brinza et al., 2020).
Conclusion
The number of studies with species of the genus Ceiba has been increased in the last decade, and this is due to the necessity of investigation on their use in folk medicine. Nonetheless, species endemic to Brazil are still poor in scientific evidence. This might be a result of their distribution, semi-arid region, where the flora is poorly studied, and the competition with other species that have economic value, often preferred targets for scientific studies.
Because of the high medicinal and pharmacological potential reported for this genus, it is necessary that new studies are carried out, in order to investigate the bioactive properties of Ceiba species not yet studied. This knowledge is fundamental for the process of preservation, validation of traditional uses, and local economy development through the bioeconomy. In addition, new studies on these plants can help in the prospection of compounds of biotechnological interest that can be applied in the most diverse industry areas, such as food, cosmetics and pharmacological.