Introduction
The natures have an abundance of secondary metabolites containing natural products, many of which exhibit various pharmacological characters. Likewise, there are unique plants, trees, herbs, shrubs, and species that have pharmacological activities and therapeutic effects (Hussein & El-Anssary, 2019). For example, Litchi chinensis, a species of edible fruits of the soapberry family, Sapindaceae, has various chemical components that have shown different pharmacological actions. It is accessible in the global market as an essential fruit with delicious fruiting bodies and nutritional value in every part of the country (Ibrahim & Mohamed, 2015).
Litchi chinensis Sonn is one of Nepal's important sub-tropical fruit crops after mango, banana and guava, an evergreen tree, is distributed in tropical and subtropical regions. In Nepal, it is widely used as an essential fruit category, traditionally having more nutritious value and also found to have hypoglycemic effects (Manandhar, 2002). The various study has suggested that the secondary metabolites of this species seeds, pericarp, fruits, leaves, bark have a various pharmacological character like antioxidant, hepatoprotective, antiviral, hypoglycemic, anti-metastatic, wound healing, hypopigmentation, antimicrobial (Ibrahim et al., 2015).
An inflammatory response is a protective response generated by the immune system to ensure survival amid infection and tissue damage. It is a protective response of the body by the variety of physical trauma, noxious chemicals or microbiological agents, which are necessary for the maintenance of the homeostasis function of the tissue. There are a variety of injuries to tissues and cells when inflammation is uncontrolled, and this can lead to chronic inflammation, long-term illness, and even cancer (Chen et al., 2017). Anti-inflammatory medicines are the standard of care for the majority of today's inflammatory illnesses, like steroidal and non steroidal anti-inflammatory drugs. However, long-term use of those drugs may lead to various drug-related adverse effects like gastrointestinal disorders, immunodeficiency, and humoral disturbances (Fokunang, Fokunang, Frederick, Ngameni, & Ngadjui, 2018; Sanders & Kraff, 1984).
Here we target to discover if the extract had anti-inflammatory agents that could be developed for long term administration. Various studies reports had suggested that L. chinensis possesses remarkable curative effects in various pathological conditions (Ibrahim et al., 2015). So to evaluate its seeds extract pharmacological potency. The anti-inflammatory and analgesic properties of LCSE were examined. It is generally established that inflammation is a complex process that begins with the recognition of a certain molecular sequence linked with infection or tissue injury. Numerous important regulators are involved in the regulation of pro-inflammatory molecule expression. Prolonged inflammation is frequently connected with serious negative health consequences (Ahmed, 2011). The anti-inflammatory properties of LCSE were tested in this work utilising in vivo animal models, namely Carrageenan-induced rat paw edoema, which was used to examine vasodilation, edoema development, tissue hyperplasia, exudates, and cellular migration. (Patil et al., 2019). Furthermore, to understand the preliminary analgesic activity of LCSE, we used Hot plate model in-vivo using animals designed for examining the response towards various pain receptors.
Materials and method
Plant materials
Mature fresh seeds of L. chinensis were gathered from the Institute of Agriculture and Animal Science's Agricultural Farm in Rampur, Chitwan, Nepal. Following collection, plant material was recognised taxonomically as L. chinensis at the Rampur Institute of Agriculture and Animal Science. Before the studies, the seeds were rinsed individually with deionized water, dried in the shade, and stored in a tightly sealed glass container. The fruit material was deposited in the laboratory of Chitwan Medical College's division of traditional Nepalese Medicine with the voucher specimen number (LC-SD-02-2017) for future reference.
Chemicals and reagents
Pure and research-grade chemicals and reagents were employed in this study. Carrageenan and standard drug diclofenac sodium were purchased from Sigma-Aldrich (USA) and N.S. Chemicals, India.
Experimental Animals and Ethics Statement
The Department of Plant Resource, Kathmandu, Nepal, provided healthy male Wistar albino rats (200-250 g) of body weight (BW). With a maximum capacity of six animals per polypropylene cage, all of the animals were housed in a single enclosure. Under a 12 h light/dark cycle, the animals were kept at a temperature of 25 ± 2 ºC and a relative humidity of 55–65 percent in a free-flowing pellet diet and water ad libitum. For two weeks prior to the experiment, all animals were acclimated to a typical laboratory environment. The Institutional review committee at Chitwan Medical College in Nepal gave ethical approval before any animal studies were carried out (Ref. No. CMC-IRC: 2073/74:100). The National Institutes of Health's Guide for the Care and Use of Laboratory Animals was followed in the care and use of experimental rats (Council, 2011).
Plant extract preparation and extract percentage yield
L. chinensis dried seed powder was pulverised in a grinder and macerated in 70% ethanol for three days at room temperature. After then, a muslin cloth and Whatman no. 1 filter paper were used for filtration. Filtrates were heated to 40 oC in a rotary evaporator to evaporate under reduced pressure (Heidolph, Germany). Finally, the obtained semisolid extract was kept in an airtight container in the freezer below 0 oC until use. To calculate the extract yield percentage following formula can be used:
Extract % yield = (Weight of dried extracts / Weight of plants sample) ˣ100
Phytochemical screening
To conduct preliminary phytochemical analysis, we used established phytochemical procedures to determine the presence or absence of bioactive metabolites in freshly generated crude LCSE. (Evans, 2002).
Oral acute toxicity assay
The acute toxicity of LCSE was measured using the techniques specified by the Organization for Economic Cooperation and Development (OECD No. 423) for evaluating the safety of research materials.(OECD, 1996). Briefly, the study plant extract was administered at a specified dose in two phases. After that, all treated animals were observed for 4 h to 72 h for the sign of general toxicity and mortality for confirming the lethal dose (LD50).
Experimental design
Before experiments, rats were fasted for sixteen hours and given access to drink ad libitum. Animals were divided into five different groups, each with six rats. Group I served as the control group (got distilled water at a concentration of 10 mL/kg), Group II served as the standard group (received diclofenac sodium at a concentration of 25 mg/kg), and Group III to V received LCSE at concentrations of 150, 300, and 600 mg/kg, respectively. The standard medication and plant extract were dissolved in 0.5 percent Carboxymethylcellulose (CMC) and delivered through oral gavage tube to the experimental animal.
Carrageenan-induced paw edema
The anti-inflammatory impact of LCSE was determined using the previously published method of carrageenan-induced paw oedema in Wistar albino rats (Dzoyem, Mcgaw, Kuete, & Bakowsky, 2017). Animals were divided randomly into five groups of six rats each. The normal control group (distilled water, 10 mL/kg), standard drug (diclofenac sodium, 25 mg/kg) and the test extract of LCSE (150, 300, 600 mg/kg) were administered orally via oral gavage tube to the respective groups. Edema was induced in the right hind paw sub-plantar region of each rat 30 minutes after treatment with 0.1 ml of 1% carrageenan solution was administered. Before and after the carrageenan injection, paw volume was measured at "0 min," "30 min," "60 min," "120 min," and "180 min." The following formula was used to calculate inhibitory activity from 30 minutes to 240 minutes after carrageenan injection:
% inhibition = Ec – Et / Ec ×100
Ec = An hourly breakdown of the edoema rate in the control group
Et= Time-dependent edoema in the experimental group
Hot Plate Test
This model evaluated analgesic potency of LCSE (Dzoyem et al., 2017). At first, hot plate temperature was maintained at 55±1oC followed by treatment of rat with distilled water 10 mL/kg or L. chinensis seed extract (150, 300, 600mg/kg) or diclofenac sodium (25 mg/kg) orally were placed on the hot plate and preventing the loss of heat by covering with a glass beaker. Cut off time was set up to 15 sec, after then latency time (responding to a licking of the forepaws or a jumping stimulus) was recorded before administration of extracts or standard drug after 0, 30, 60, 120, and 180 min of extract /standard drug administration.
Maximum possible analgesia (MPA) = [(Rt-Rc)/ (30 sec - Rc) ×100]
Where, iRt= iReaction time for treatment, iRc= iReaction time for control
Statistical Analysis
Mean ± SEM was used to express the outcomes of the experiments. ANOVA and Tukey's post hoc tests were used to compare the differences between treatment groups. p<0.05 was used as the threshold for statistical significance. All statistical tests were performed using SPSS version 16.
Results and Discussion
Phytochemical screening and extractive yield percentage
LCSE shows the positive result detecting the presence of phenols, tannins, flavonoids, saponins, steroids and alkaloids. The extraction yield percentage was found in the LCSE (3.336%) with dark brown crystal type extracts.
Acute toxicity study
When Rat is given orally up to 5000 mg/kg, the ethanol extract of seeds of L. chinensis, no adverse effect like lacrimation, excitation, frequent urination, skin irritation was observed except mild sedation and analgesia at all tested doses. However, no mortality was seen in any of the above doses at the end of 72 hr of observation.
Anti-inflammatory activity by Carrageenan induced paw edema
The in vivo anti-inflammatory activity of LCSE was compared with standard drug Diclofenac sodium and control at 30 minutes and in 60 minutes difference up to 240 minutes after the induction of inflammation. The result of anti-inflammatory activity was expressed as mean ± standard error of the mean of inflammation index as shown in Table 1.
Table 1
Effect of LCSE at 150, 300 and 600 mg/kg between doses on carrageenan-induced paw oedema in rats.
The percentage inhibition of rat paw edema by standard diclofenac and test extract was observed, as shown in Table 2.
Table 2
Percentage inhibition of rat paw edema volume by LCSE concerning diclofenac sodium.
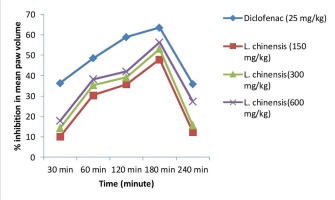
Similarly, Figure 1 demonstrates LCSE and standard drug Diclofenac percentage inhibition of rat paw edema. At 180 minutes, L. chinensis (600 mg/kg) showed 56.25% inhibition of rat paw edema concerning the standard Diclofenac (25 mg/kg). LCSE showed a significant decrease in rat paw edema from 30 minutes to 240 minutes at different study doses.
Figure 1
Percentage inhibitions of rat paw edema volume of different dose of LCSE with reference to diclofenac sodium.
Various synthetic drug development has been carried out in a world, even though this many of them are found to have some or other side effect, but when it comes to about analysis of plant-derived medicinal product which has held it own unique place by defining no side effects or too little side effects which is incomparable with the side effects shown by synthetic drugs (Koparde, Doijad, & Magdum, 2019).
Carrageenan is a sulphated polysaccharide obtained from seaweed and is commonly used to induce acute inflammation, and is believed to be biphasic for evaluating novel anti-inflammatory compounds (Coura et al., 2015). During the early stages of inflammation, histamine and serotonin are released, while during the latter stages, bradykinin, Protease, Prostaglandin, and lysosomes are released (Abdulkhaleq et al., 2018).
As a traditional medicine tonic for the brain, heart, liver and skin, Litchi chinensis plant components have been employed (Kilari & Putta, 2016). Various Scholar has reported that various part L. chinensis have anti-inflammatory potency, antidiabetic, antioxidant action (Ibrahim et al., 2015). Administrations of different doses of seed extract of L. chinensis significantly reduce carrageenan-induced paw edema at different time intervals, which is concentration-dependent. The maximum percent of inhibition of paw edema was observed at 3hr with a dose of 600mg/kg, which is 56.25% by seeds extract of L. chinensis.
As flavonoids containing compound has been reported to normalize cellular activities of the inflammation-related cell (Ginwala, Bhavsar, Chigbu, Jain, & Khan, 2019). Saponins also have been reported to attenuate the expression of TNF-a, IL -1B, IL-6, proinflammatory cytokines (Jang et al., 2016). Preliminary studies have concluded that ethanolic extract of leaves of L. chinensis possesses a positive test for the occurrence of flavonoids and saponins.
Analgesic activity by Hot plate test method
The analgesic activity of LCSE was evaluated in rats using the hot plate test method.L. chinensis extract at different doses and standard drug diclofenac was found to exhibit significant activity compared to the control group. LCSE showed significant analgesic activity during all the reaction time, as shown in Table 3 and the maximum possible analgesia percentage in Table 4.
Table 3
Analgesic activity of LCSE via Hot plate method.
Data are presented as mean ± SEM (n = 6 in each group). *p<0.001 was considered as significant when compared to the control group.
Table 4
Maximum possible analgesic (%) of LCSE concerning standard diclofenac sodium.
Date are presented as mean ± SEM (n = 6 in each group). *p<0.05, **p<0.001 was considered significant and n.s = non-significant when compared to Diclofenac sodium as standard.
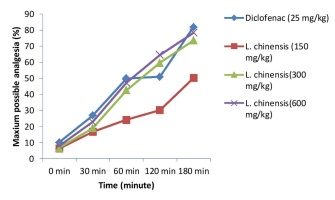
Maximum possible analgesia, 64.67% for a dose of 600 mg/kg followed by 59.61% for a dose of 300 mg/kg at 120 min, which is statistically significantly different (p<0.001) from the standard drug Diclofenac. LCSE at the dose of 600 mg/kg at 180 min showed the highest possible analgesia activity, i.e. 78.47% (Figure 2).
Due to injuries or illnesses, nociceptors are responsible for sending information to the central nervous system (CNS) identifying where and how intense the pain is (Garland, 2012). The hot plate method is a popular approach for testing analgesics that act on the peripheral nervous system (Mishra, Ghosh, Kumar, & Panda, 2011). Administration of different dose of seeds extract of L. chinensis significantly delay the latency time of animal to the heat stimulus in the hot plate test model, which is concentration-dependent, maximum percent of analgesia, is observed at 3hr with dose 600mg/kg which is 78.47% by seeds extract of L. chinensis as flavonoid and tannin-containing compound have been reported to produce analgesic activities by the leaves of L. chinensis (Zhang et al., 2015).
Conclusions
The present study concludes that 70% ethanolic seed extract of L. chinensis possesses a positive test for terpenoids, flavonoids, phenols, tannins, and saponins. The LCSE was found to be safe up to a dose of 5000 mg/kg orally in acute toxicity testing. At 150, 300, and 600 mg/kg, the oral administration of LCSE had a significant anti-inflammatory and analgesic effect. Molecular-level investigations could be carried out to understand the specific mechanism of action by which extracts exert their analgesic and anti-inflammatory impact and to identify the bioactive ingredients responsible for this activity.
Ethical approval
Before performing any animal research, the Chitwan Medical College Institutional Review Committee (Ref. No. CMC-IRC: 2073/74:100) in Nepal got ethical approval.
Author contributions
Concept and experimental design: 1SKC, BP; Performed the experiments: 1SKC, BP, SG, 2SKC; Data analyzed and analysis tool: 1SKC, BP; Writing of the manuscript: 1SKC, 2SKC, SG; Supervision: AG; Revised manuscript critically for important intellectual content: AG, BP.
1SKC - Sindhu KC; 2SKC - Sistu KC