Introduction
Flavonoids constitute a large group (>8000 independent known compounds) of natural polyphenolic phytochemicals mainly in all kinds of plants and are essentially present in the human diet. Natural flavonoids have multiple roles: screening of light in plants, antioxidants, anti-allergic, antimicrobial, anti-inflammatory, photo-reception, impart colours to attract the vision, and insect repellents (Pietta, 2000; Ren, Qiao, Wang, Zhu, & Zhang, 2003) . Initially, the studies have focused on in vitro but not the in vivo antioxidant properties of the flavonoids mainly because of their quick scavenging reactive oxygen species (ROS) and shown maximum structure-activity relationship (SAR) to cope with the highest removal of free radicals (Pietta, 2000; Rice-Evans, Miller, & Paganga, 1996) . Few in vivo studies of the utilization of flavonoids are linked with limited data availability in humans because the dietary flavonoids are converted into degraded products such as phenolic acids, which still behave the potential scavengers. Moreover, flavonoids are widely used in chemoprevention and chemotherapies to cure tumors for centuries, mainly in traditional medication systems (Cushnie & Lamb, 2005; Ren et al., 2003) . Certain experimental studies demonstrated that dietary flavonoids also help reduce cardiovascular issues, coronary heart disease, stroke, and atherosclerosis (Cook & Samman, 1996; Diego et al., 2020; Peterson & Dwyer, 1995; Terao, Kawai, & Murota, 2008; Wu et al., 2020). Furthermore, the antimicrobial activities of such flavonoids mainly depend on the structure of the compounds. Flavonoids inhibit the activation of microbes mainly by a vital check on DNA gyrase enzyme action, interfere with the cytoplasmic membrane functions, and alteration in the energy metabolism of the microbial cell (Cushnie et al., 2005).
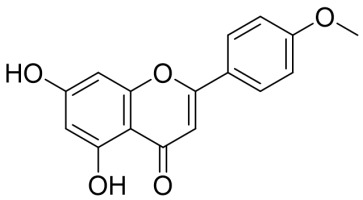
The acacetin (5,7-dihydroxy-4'-methoxy flavone) (Figure 1) is a well-known flavonoid, which initially was extracted as the main active constituents from many plant species such as Agastache rugosa (Lamiaceae) plant (Cho et al., 2014), Robinia pseudoacacia (Fabaceae) (Ha et al., 2012), Saussurea involucrate (Asteraceae) (Liou et al., 2017), Turnera diffusa (Passifloraceae) (Fan et al., 2015), Echinochloa esculenta (Poaceae) (Lee et al., 2017), Cirsium rhinoceros Nakai (Asteraceae) (Kim, Cha, Li, & Cha, 2015), and Calea urticifolia (Asteraceae) (Chaurasiya et al., 2016). Acacetin is a 4′-O-methylated flavone of apigenin, the parent compound, and in various plants, species, synthesize an enzyme apigenin 4′-O-methyltransferase that utilizes S-adenosyl methionine and 5,7,4′-trihydroxyflavone (apigenin) to produce S-adenosylhomocysteine and acacetin. Moreover, (Robinson & Venkataraman, 1926) had successfully prepared acacetin over 100 years ago, and by this period, dozens or hundreds of modifications have been prepared from this compound, such as a recent synthetic preparation (Hanamura, Hanaya, Shoji, & Sugai, 2016).
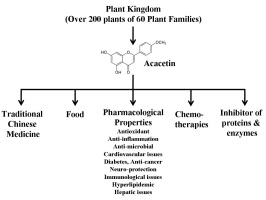
Acacetin is cosmopolitan in distribution within the plant kingdom. It is reported in more than 200 plant species of 60 plant families, but most plants belong to the Asteraceae and Lamiaceae families, including Artemisia, Cirsium, Dendranthema, Saussurea, Dracocephalum, and Origanum. The main isolation techniques described for acacetin from these over 200 plant species are liquid chromatography-mass spectrometry (LC-MS), liquid-liquid extraction (LLE), nuclear magnetic resonance (NMR), thin-layer chromatography (TLC), paper chromatography (PC), capillary electrophoresis (CE), high-performance liquid chromatography (HPLC), gas chromatography (GC), and Gas chromatography-mass spectrometry (GC-MS). Moreover, HPLC combined with electrospray ionization quadrupole time of flight tandem mass spectrometry (HPLC-ESI-QTOF-MS/MS) has also been applied in recent studies. In-depth details of these extraction methods are given in the subsequent headings. In the Traditional Chinese Medicine (TCM) system, the crude extracts of plants having acacetin remained a frequent remedy to cure multiple diseases. Acacetin is reported to possess numerous biological properties, including anti-inflammatory (Singh, Gupta, Meena, & Luqman, 2020), antioxidant properties, neuroprotection (Cho et al., 2014; Ha et al., 2012; Tanigawa et al., 2013), antiproliferative (Ha et al., 2012; Pan, Lai, Wang, & Ho, 2006), anticancer (Punia, Raina, Agarwal, & Singh, 2017; Singh et al., 2020; Wang et al., 2020), and lot more. In anti-inflammatory conditions, acacetin mainly modulates the suppression of phosphorylation of p38 mitogen-activated protein kinases (MAPKs) and nuclear factor (NF)-κB (Tanigawa et al., 2013; Wang, Jiang, Pang, Zhou, & Gu, 2020). In addition to these, acacetin also helps reduce lipid peroxidation through its active ROS scavenging capability and revives against hypoxia-induced neonatal cardiomyocyte injury (Yang et al., 2014). The drug acacetin has diverse biological and pharmacological properties (Semwal et al., 2019), detailed below (Figure 2).
Extraction Techniques of Acacetin
Acacetin is the aglycone of the flavonoid glycoside linarin (5,7-dihydroxy-4'-methoxy flavone-7- O-β-D-rutinoside), which occurs naturally 100s of plant families, but mainly in Asteraceae and Lamiaceae. The details of all these plants from which acacetin is being extracted and the identification methods have been summarized and found mainly in Artemisia, Cirsium, Dendranthema, Saussurea, Dracocephalum, and Origanum. Because of its promising pharmacological activities, massive data on acacetin exploration, isolation, separation, and finally purification from various plants or herbal medicines have been reported extensively using conventional separation tools such as liquid-liquid extraction (LLE), column chromatography and HPLC, which can meet the minimum separation requirements of purity for NMR identification.
Methods of extraction of acacetin from Chrysanthemum species include direct acid hydrolysis by uniform design method (Zhu, Wang, Lian, Jin, & Li, 2012). Researchers used supercritical CO2 extraction flavonoids included acacetin from rosemary and sage (Ivanović et al., 2009). Recently, a new extraction method, namely temperature-responsive ionic liquids, for the simultaneous extraction of hydrophilic and lipophilic phenolic compounds from Chrysanthemum species (Wang, Wang, Li, & Yang, 2019). Using an ultrasound-assisted approach in conjunction with a central composite circumscribed (CCC), another method for optimising phenolic chemicals from Dendranthema indicum was developed and implemented. Optimal solvent conditions for this CCC approach were determined to be ethanol and acetic acid (70:20, v/v), with the temperature set at 57°C, a solid-to-liquid ratio of 1:30 g/mL, and an extraction period of 20 minutes. (Zhong et al., 2019). An exact microwave-assisted extraction method is designed to extract acacetin from Veronicastrum latifolium (Hemsl.). In order to extract and enrich flavonoids, Yamazaki used microwave irradiation followed by dispersive solid-phase extraction as a sample preparation method. This approach was developed using single-factor and Box–Behnken experiments. (Yin, Zheng, Wang, & Wang, 2019).
Analytical Techniques of Acacetin
Methods including TLC, PC, GC-MS, HPLC, HPLC-ESI-QTOF-MS/MS, and CE were developed for flavonoid sample analysis containing acacetin. The GC-MS technique analyzes the volatile oil constituents from Sonchus arversis L. has reported acacetin as the main constituents account for 0.94% of total volatile oil (C & N, 2008) . Several recent studies used the CE method for determining flavonoids in various plant species and detected the occurrence of acacetin in all samples tested (Chu, Jiang, & Ye, 2010; Peng, Yuan, Liu, & Ye, 2005; Wu, Yu, & Li, 2017; Yu, Chu, & Xu, 2011). A highly specified but sensitive electrospray ionization liquid chromatography-tandem mass spectrometry method (EILC-TMS) is recently designed to explore flavonoids and phenolics in Chrysanthemum, Artemisia, Dendranthema and Origanum species (Hossain, Rai, Brunton, Martin-Diana, & Barry-Ryan, 2010; Li et al., 2019; Tahri et al., 2016; Zhong et al., 2019; Zhou et al., 2010). These techniques have been well recognized and can thus be used to analyse and explore acacetin in various related plant species.
Pharmacological Activities
Anti-microorganism activity
Multi-drug resistance (MDR) is a growing public health concern worldwide. Recent developments in molecular biology led to a renewed interest in the binding mechanism of the MDR strains of microorganisms. Data from several studies suggest that many surface proteins play a vital role in bacterial virulence (Cascioferro, Cusimano, & Schillaci, 2014; Scott & Barnett, 2006). These proteins are bound covalently to the core structure of the bacterial cell walls by sortase enzyme (SrA) (Bi et al., 2016), especially in Staphylococcus aureus. Extensive research has shown that SrA is the potential target for attenuating S. aureus virulence.
The antimicrobial activity of acacetin was investigated in a rat model challenged with S. aureus infection. It was observed from the results that acacetin inhibits the catalytic activity of SrA by establishing a compact conformation binding with SrA via residues Arg-139 and Lys-140. Recent studies suggesting acacetin could be used in the preparation of potential anti-S. aureus drugs (Bi et al., 2016). In vitro antiviral effect of acacetin and some other flavonoids were investigated on Herpes simplex type 1 (HSV-1) and found to be the potent antiviral agent among all other flavonoids its action in a concentration-dependent way. However, the virucidal activity was weak at a higher dose of acacetin. The intense inhibitory action on the protein synthesis in virus-infected cells was responsible for its antiviral activity (Hayashi, Hayashi, Arisawa, & Morita, 1993).
Effect on the cardiovascular system
Data from certain experimental studies demonstrated that dietary flavonoids have variable effects on cholesterol to help retard cardiovascular issues and atherosclerosis (Terao et al., 2008). For instance, atherosclerosis occurs due to higher oxidized low-density lipoproteins (LDL) accumulations in the macrophages (Terao et al., 2008). The flavonoids and their metabolites in the blood system reside on the plasma albumin fraction (PAF) but not on LDL fractions, and the level of active flavonoids on the former location help decide the anti-oxidative effect in in-vivo (Terao et al., 2008). Moreover, high-density lipoproteins (HDL) showed the athero-protective effects by directly lessening the recurrence of atherosclerosis (Millar, Duclos, & Blesso, 2017; Terao et al., 2008). However, in particular inflammatory conditions, HDL becomes pro-atherogenic and dysfunctional, while active flavonoids along with their metabolites residing on PAF help improve the efficacy of the HDL particles in the bloodstream by regulation of reverse cholesterol transport in macrophages and the modulation of hepatic paraoxonase 1 expression (Millar et al., 2017).
Moreover, flavonoids regulate HDL antioxidant and cholesterol efflux capacities in patients with diabetes, hypertension, and hyperlipidemic issues (Millar et al., 2017). Despite this, many flavonoids have not been studied for their physiological effects, and hence drugs directly targeting HDL activity are yet to be explored. Hypercholesterolemia is a condition due to an increased level of LDL, which is the main factor leading to developing atherosclerosis and myocardial ischemia (Saini, Arneja, & Dhalla, 2004). Moreover, hypercholesterolemia mediated cardiovascular dysfunction is mainly because of altered enzyme actions, cellular membrane fluidity, and ionic transport in cardiomyocytes, while increasing oxidized cholesterol byproducts lead to atherosclerosis (Saini et al., 2004).
The atrium-selective anti-arrhythmic effect of acacetin using ultrarapid delayed rectifier K current (IKur) and various cardiac ionic currents was investigated and found that acacetin has excellent potential to inhibit the atrial fibrillation and extend the atrial effective refractory duration without delaying the edited QT period after intraduodenal administration (Li et al., 2008). Moreover, the anti-arrhythmic activity of acacetin was observed by inhibiting the Ca2+-activated potassium (KCa) currents on small conductance firmly expressed in HEK 293 cells (Chen et al., 2017) . In another experimental study, acacetin showed the inhibitory activity on the open hKv1.5 channels by adhering to the S-6 domain, indicating the anti-arrhythmic potential of acacetin in human atria (Wu et al., 2011) . Similarly, the molecular behaviour of acacetin for blocking hKv4.3 channels continuously expressing in HEK 293 cell line, and found the inhibitory action on the closed channels by adhering to its respective P-loop filter helix and S-6 domain (Wu et al., 2013) . This unique feature may help manage arterial fibrillation. The synergistic anti-arrhythmic effects of acacetin with Na blockers by the combined blockage of atrial-specific IKur and Na current, and supposed to be used while treating the atrial fibrillation (Ni et al., 2017). In cardiac remodelling via MAPK and P13K/Akt signaling pathways after inducing myocardial infarction in mice., acacetin also showed protective effects (Chang et al., 2017).
Recently acacetin showed inhibitory effects on rat cardiomyocytes during ischemia/reperfusion-induced reduction of anti-oxidative stress-responsive proteins, e.g. thioredoxin and superoxide dismutase-2 (SOD2) (Liu et al., 2016) . Acacetin reduces lipid peroxidation while boosting antioxidant activity to pose cardio-protective effects in myocardial ischemic rats (Yang et al., 2014). Moreover, acacetin also stimulates the AMPK-mediated NF-E2-related factor 2 (Nrf2) activation in the cardiomyocytes protection in neonatal rats (Wu et al., 2018). Similarly, Liu and colleagues (Liu et al., 2016) found that acacetin protects the ischemic heart injury when administered intravenously at 10 mg/kg of body weight dose by diminishing the ventricular arrhythmia score and total time duration, reducing the ventricular fibrillation and infarct size to improve the improper functioning of the heart in anaesthetized rats (Liu et al., 2016).
Effect on the central nervous system
Acacetin is suggested to alternate in brain disease involving neuro-inflammations (Yu, Cai, Kang, Zhang, & Zhou, 2012). During certain neurological disorders, and increased glutamate level has been reported to be linked with these disorders. Extensive studies were conducted using acacetin to find its effects on the nervous system. Acacetin inhibits Kv1.3 Channels and Human T cell activation, indicating immunomodulatory and anti-inflammatory actions in vitro (Zhao et al., 2014). Microglia under over-active conditions secretes certain proinflammatory and cytotoxic factors which lead to Alzheimer’s disease, Parkinson’s disease and ischemia, and acacetin is reported to regulate these neuroinflammatory factors (proinflammatory cytokines, tumor necrosis factor α (TNFα), interleukin 1β (IL-1β), NFκB and p38 MAPK) and hence suppress the early onset of these diseases (Ha et al., 2012). Acacetin (5,7-dihydroxy- 40-methoxy flavone) isolated from Robinia pseudoacacia, showed an inhibitory effect on the production of nitric oxide (NO) and prostaglandin E2 and reduced the expression of inducible NO synthase and cyclooxygenase 2 (COX-2) both in vivo and in vitro (Ha et al., 2012; Pan et al., 2006).
Moreover, in vivo results showed a colossal suppression of microglial activation, reducing the apoptosis in neuronal cells, and hence it would appear a potential applicant for curing neuroinflammatory diseases. Endogenous glutamate levels in vivo are regulated by Acacetin, which acts as an inhibitor of depolarization-evoked glutamate release and controls the concentration of cytosolic free Ca2+ in the hippocampus nerve terminals (Lin, Huang, Wu, Lu, & Wang, 2014). Acacetin also inhibits glutamate release from hippocampus synaptosomes by reducing voltage-dependent Ca2+ entry in a kainic acid rat model. (Lin et al., 2014). Acacetin protects 6-hydroxydopamine neurons (6-OHDA) -induced neuronal cell death via mitochondrial apoptosis (Kim et al., 2017). Acacetin affects the inflammatory corpuscle 3 (NLRP3) after cerebral ischemia-reperfusion injury in a rat model was investigated. It was found that acacetin possesses a neuroprotective effect by inducing the NLRP3 signaling pathway (Bu et al., 2019). In another study, acacetin has excellent potential to protect dopaminergic (PD) neurons against the neurotoxicity involved in PD via its anti-inflammatory action (Kim et al., 2012).
Anti-inflammatory and organ-protective effect
This inhibition of the MAPK/Akt/NF-κB pathway and reduced ROS mainly suppress the inflammations within the atherosclerotic lesions and modulates immune responses (Choi et al., 2010). Various flavonoids mimic similar results were reported extensively (Céspedes, Alarcon, Avila, & Nieto, 2010). Moreover, in RAW 264.7 cells, acacetin inhibits LPS-induced up-regulation of (COX-2) and nitric oxide synthase (NOS). Acacetin showed good potential to down-regulate the COX-2 and inhibitory nitric oxide synthase (iNOS) expression by modulating the NF-κB activation suggesting that acacetin is a superior drug to prevent inflammation associated conditions (Pan et al., 2006). Acacetin also showed anti-arthritic activity in fibroblast-like synoviocytes and inhibited the p38 and c‐Jun NH2‐terminal kinase (JNK) phosphorylation, reducing matrix expressions metalloproteinase (MMP)-1, MMP-3 and MMP-13. Hence acacetin appears as an alternative in rheumatoid arthritis (Chen, Yang, Hu, Bao, & Wu, 2015). Acacetin also showed anti-arthritic activity in fibroblast-like synoviocytes and inhibited the p38 and JNK phosphorylation, reduces the expressions of matrix metalloproteinase (MMP)-1, MMP-3 and MMP-13, hence acacetin appears as an alternative in rheumatoid arthritis (Fan et al., 2015).
The adipocytes of obese mice showed that acacetin has inhibitory effects on adipogenesis, and inflammatory responses of macrophages stimulated using different culture mediums from 3T3-L1 cells (Liou et al., 2017). In addition to these, acacetin also helps reduce lipid peroxidation through its active ROS scavenging capability and revive against hypoxia-induced neonatal cardiomyocyte injury (Yang et al., 2014). Acacetin also decreased the generation of inflammatory mediators in IFN-γ-activated murine macrophages. The anti-inflammatory actions of acacetin have a specific positive effect on acute lung injury (ALI). Acacetin showed higher antioxidant activity in LPS-mediated acute lung injury by diminishing oxidative stress and activation of oxidative responsive genes viz. heme oxygenase 1 (HO1), superoxide dismutase (SOD), and inducible nitric oxide synthase (iNOS) in mice (Wu, Wang, Zhang, Du, & Li, 2018). Acacetin is quite active in alleviating D-galactosamine/LPS-induced liver injury through suppressing the toll-like receptor-4 (TLR4) signaling pathway (Cho et al., 2014). Acacetin and apigenin rich extracts of Artemisia sacrorum Ledeb. (Asteraceae) synergistically inhibits lipid accumulation in 3T3-L1 cells both at transcriptional and translational levels by reducing the expression of genes and proteins, which is linked with lipogenesis and adipogenesis (Ma et al., 2018). This synergistic composition shows a significant reduction in lipid accumulation and triglyceride levels compared with a single application of apigenin or acacetin (Ma et al., 2018). When LPS and GalN were used to produce full-blown hepatic failure in mice, the hepatoprotective effects of acacetin were more pronounced, principally due to the suppression of TLR4 signalling and increased autophagic flux (Cho et al., 2014). Kidney histopathological and functional criteria improvements of in vivo model examinations showed that acacetin effectively recovered renal ischemia-reperfusion damage when applied dose-dependently, mainly by its antioxidant activity, reduced MDA levels, and apoptotic cell deaths (Shiravi, Jalili, Vaezi, Ghanbari, & Alvani, 2020).
Acacetin poses potential anti-inflammatory effects in vitro, while orally applied acacetin pose anti-inflammatory effects in ovalbumin-(OVA-) sensitized asthmatic mice, and where it lowers IL-6, IL-8, intercellular adhesion molecule-1 (ICAM-1), and eotaxin-1 (Huang & Liou, 2012). Additionally, acacetin significantly reduced eosinophils' propensity to associate with inflammatory BEAS-2B cells, implying that dietary acacetin reduces asthma symptoms in OVA-sensitized mice. (Huang et al., 2012).
Antipyretic and antinociceptive activity
Fever and pain management are exigent for the clinician worldwide as the existing treatment with available drugs often accompany specific side effects. The chloroform extracts of Potentilla evestita and its main constituent acacetin in a dose-dependent manner had shown intense antipyretic and antinociceptive activity in various in vivo studies (Rauf, Khan, Khan, Ullah, & Pervez, 2014). The possible mechanism of antinociceptive activity of acacetin by the involvement of 5-HT1A, GABA/BDZs and opioid receptors in the rat model were also elaborated recently (Carballo-Villalobos, González-Trujano, & López-Muñoz, 2014). Agastache Mexicana plant extract is a good source of acacetin and may be used as an alternative treatment for visceral pains due to its intense antinociceptive action (González-Trujano, Ventura-Martínez, Chávez, Díaz-Reval, & Pellicer, 2012).
Effect on blood glucose system
It is said that during normal physiological conditions, blood glucose levels are under tight control. Various plant extracts, specially Anoda cristata (L.) Schltdl. reported enriched with acacetin have been applied to cure diabetes (Juárez-Reyes et al., 2015). Moreover, acacetin mainly inhibits aldose reductase activity directly linked with diabetic retinopathy (Shin, Kang, Seo, & Shin, 1995). In diabetic mice, acacetin (3 and 31.6 mg/kg) declined hyperglycemic by lowering blood glucose levels (Juárez-Reyes et al., 2015). Acacetin acts through binding with the active site of the aldose reductase enzyme, disrupting protonation by hydrogen bond formation with Tyr48 of this enzyme, which is thought to be a fundamental regulator of diabetes-mediated oxidative stress retinopathy (Manivannan, Soundararajan, Park, Sakkiah, & Jeong, 2015).
The flower extracts of Chrysanthemum sinense SABINE possess acacetin and 4,5-O-dicaffeoylquinic acid methyl and are applied on hyperglycemic mice produced using uricase inhibitor potassium oxonate, and acacetin reduces uric acid levels in hyperglycemic mice serums (Nguyen et al., 2005). It is suggested that the hypouricemic potential of acacetin is due to its capability for inhibition of xanthine oxidase activity (Nguyen et al., 2005).
Antiproliferative activity or anti-cancer activity
Flavonoids are a highly dispersed group of phytochemicals, almost found in every class of higher vascular plants and gives unique colors to the various parts of the plants. These flavonoids are potent antioxidants naturally present in almost every food and potentially inhibits carcinogenesis. Identifying the primary molecular targets of these phytochemicals is crucial for selecting anti-cancer drugs before the treatment.
Acacetin affects human liver cancer HepG2 cell proliferation by regulating apoptosis and arrest cell cycle progression in the G1 phase (Hsu, Kuo, & Lin, 2004). Acacetin plays a significant role in increasing p53 and p21/WAF1 proteins, which contributes to cell cycle arrest (Hsu et al., 2004). Moreover, acacetin-mediated apoptosis of HepG2 cancer cells followed the enhanced expression of Fas/APO-1 (membrane-bound and soluble Fas ligand) and BCL2-associated X protein (Bax protein) (Hsu et al., 2004).
On NSCLC cells, the synergistic effect of acacetin and doxorubicin was explored. Acacetin improves doxorubicin retention in cells via altering drug transporters.This feature supports the therapeutic use of acacetin in cancer patients (Punia et al., 2017). Acacetin's potential effect on cytoprotection and proliferation in human gastric cancer AGS cells was explored, and it was discovered that acacetin suppressed cell proliferation via inducing poly (ADP-ribose) polymerase degradation of DNA fragmentation factor (DFF-45) and caspase-3 activity. Additionally, acetin causes apoptosis in a dose- and time-dependent manner. These findings suggest the pivotal potential of acacetin in cancer treatment (Pan, Lai, Hsu, & Wang, 2005).
Similarly, acacetin exhibited its antiproliferative action on human T cell Jurkat cells by attenuating the Fas-mediated pathway (Watanabe, Kanno, Tomizawa, Yomogida, & Ishikawa, 2012). In another study, the underlying mechanism of how acacetin induces apoptosis in human breast cancer MCF-7 cells was studied, it was found that acacetin mediates the activation of ROS generation, cellular cascades like SAPK/JNK1/2-c-Jun signaling pathway, and mitochondria-mediated cellular apoptosis (Shim et al., 2007). Similarly, the antiproliferative activity of acacetin in human non-small cell lung cancer A549 cells was investigated, and it was found that apoptotic system p53 and Fas/FasL may contribute to exhibit the antiproliferative action of acacetin in cancer cells (Hsu, Kuo, Liu, & Lin, 2004).
The transformation of E-selectin-mediated adhesion of leukocytes or cancer cells into the vascular endothelium is supposed to be a primary step to initiate the inflammatory process or metastasis. In a study, acacetin was investigated for its possible effect on the E-selectin expression in human umbilical vein endothelial cells. Acacetin remarkably reduced the expression of E-selectin by activation of NF-κB and regulation of the p38 MAPK signaling pathway (Tanigawa et al., 2013). In an experimental study, the anti-angiogenic efficacy of acacetin was observed in vitro, ex vivo and in vivo models. Acacetin also prevents Stat signaling and suppress angiogenesis in all the tested three models. It can thus be recommended that acacetin could be a potent drug to limit angiogenesis in cancer (Bhat et al., 2013).
In another study, the potential of acacetin on metastasis in human NSCLC A549 cells was investigated and found that acacetin suppresses the P38α MAPK signaling pathway, thereby checks the attack and movement of cancer cells (Chien et al., 2011). Moreover, recently, acacetin has been involved in modulating the huge protein kinases, which are linked with the genes involved in carcinogenesis, angiogenesis, cell invasion and motility, proliferation, and survival (Singh, Meena, Luqman, & Meena, 2021). Similarly, acacetin reduces the HIF-1α and VEGF expressions by targeting the protein kinase B (PKB), also called AKT and hypoxia-induced factor 1 (HIF-1) downstream regulation in ovarian cancer cells (Liu et al., 2011). Besides, the anti-metastatic activity of acacetin was also reported in human prostate cancer DU145 cells by inactivating the p38 MAPK signaling cascade (Shen et al., 2010). These results provide further support for the hypothesis that acacetin may be used to check and treat tumors.
The cytotoxic effects of acacetin are studied in detail. Acacetin causes cytotoxicity and activates apoptotic pathways involving caspases in Jurkat T cell clones (J/Neo cells). Moreover, Lee and colleagues found that acacetin initiates apoptosis and cytoprotective autophagy in Jurkat T cells simultaneously, later followed by the Akt-mTOR pathway (Lee et al., 2017). Moreover, pharmacological inhibition of autophagy in Jurkat T cells may activate the expression of Bak, which results in mitochondrial damage-driven apoptosis (Lee et al., 2017) .
Acacetin showed in vivo and in vitro partial and selective anticancer effects in chronic lymphocytic leukemia (CLL), mainly targeting cancerous mitochondria and ROS formation (Salimi et al., 2016). In hepatocellular carcinoma (HCC), overexpression of retinoic acid receptor-γ (RARγ) has been observed that lead promote tumorigenesis mainly by physical interaction with p85α regulatory subunit of PI3K and finally activates AKT (Zeng et al., 2017). Acacetin binds with RARγ to stop the activation of AKT and hence resumes activation of normal p53 signaling by maintaining the balance between p53 and AKT, which cause cancer cells to apoptosis by antagonizing the non-genomic effect of RARγ on AKT and p53 (Zeng et al., 2017).
In a recent study, acacetin was used to find the mechanism of how it induces apoptosis in the oral squamous cell carcinoma cell line (HSC-3). These effects of acacetin are mainly by anti-peroxidative, anti-inflammatory, anti-plasmodial, and antiproliferative activities that induce apoptosis (caspases pathway) and block cell cycle progression (Kim et al., 2015). Moreover, acacetin-mediated apoptosis in HSC-3 is by activating the MAPK-mediated signaling pathways with the subsequent induction of mitochondria- and caspases-dependent mechanisms (Kim et al., 2015). Acacetin significantly affects the suppression of epidermal growth factor (EGF)-induced cell transformations, i.e., phosphorylation of Akt and p70S6K, downstream effectors molecules of PI3-K (Jung et al., 2013). Furthermore, binding assay and computational data suggested that acacetin inhibits PI3-K activity by directly adhering using adenosine triphosphate (ATP)-competitive inhibition of PI3-K, and hence proven as a potential agent for the chemoprevention of melanoma (Jung et al., 2013) . Furthermore, acacetin has significantly reduced SK-MEL-28 tumor growth and Akt phosphorylation in vivo (Jung et al., 2013) .
Anti-aging Effects
The human telomeres actively silence the neighbouring or adjoining genes called telomere positioning effect (TPE), mainly regulated by various drugs that alter telomeres. Acacetin and chrysin are two well-known flavonoids that help alleviate TPE and deprotect telomeres from DNA damage responses in human cells (Boussouar et al., 2013). However, telomere deprotection triggered by protecting dysfunctions does not affect TPE. Moreover, acacetin and chrysin target multiple functions of telomeres (Boussouar et al., 2013).
Acacetin was evaluated against age-related disorders in a Caenorhabditis elegans model system and found a modulation of the up-regulation of stress-responsive genes (gst-4 and sod-3). The most striking result from the data is that acacetin promotes longevity by maintaining the tested worms' stress levels and health span. This surprising outcome suggests that acacetin may be used to develop novel therapies to manage age-related disorders in the future (Asthana, Mishra, & Pandey, 2016).
Neurodegenerative Diseases
Alzheimer’s disease (AD) is a neurodegenerative disorder, and acacetin has been reported to have great potential to hunt β-amyloid (Aβ) cleaving enzyme (BACE-1), which is a prime target during the treatment of AD patients (Wang, Perumalsamy, Kwon, Na, & Ahn, 2015). Varying concentrations (100, 300, and 500 μM) of acacetin lowered Aβ formation by interacting with BACE-1 activity and amyloid precursor protein (APP) expression. This results in reduced production levels of the APP carboxyterminal fragments along with intracellular domains of APP. Acacetin exerts its positive and modulatory effects on Aβ production by regulating BACE-1 and APP at transcriptional levels and resulting in decreased protein expressions of APP and BACE-1 (Wang et al., 2015).
In certain psychiatric and neurological diseases, the plant extracts having acacetin are known to be given. The underlying mechanism of action and healing of acacetin in certain neurodegenerative conditions is its inhibitory effects on the monoamine oxidase (MAO-A & B) (Chaurasiya et al., 2016). Among the two isoforms of the MAO (A & B), acacetin showed a preference for MAO-B due to its higher binding energy (Lee et al., 2017a) and potential for elicit selective pharmacological effects helping to treat certain psychiatric or neurological conditions (Chaurasiya et al., 2016). The molecular docking simulations confirm the preference of acacetin to binding with MAO-A and MAO-B and clarifies the binding energy difference dictates the pharmacological effects of acacetin (Lee et al., 2017). In another study, Agastache rugosa leaves were analyzed and reported five new derivatives of acacetin, which shows MAO inhibitory activities (Lee et al., 2017). Among the isolated compounds, acacetin 7-O-(6-O-malonylglucoside (AMG) and acacetin potentially but reversibly inhibits both human MAO-A and MAO-B, while Tilianin (Acacetin-7-O-glucoside) had little inhibitory activity for both isoforms of MAO (Lee et al., 2017). Both AMG and acacetin are reported as potential candidates while selecting and designing specific MAO inhibitors.
Effect on Pharmacokinetic Parameters
The pharmacokinetic study of acacetin was performed following a single intravenous administration at 5 mg/kg in rats. The maximum plasma concentration (Cmax) was 1334.9 ± 211.6 ng/mL using UPLC-MS/MS technique. Acacetin concentration in plasma was rapidly declined, initially suggesting the rapid distribution of the compound to other tissues. Acacetin was eliminated from the body with a terminal half-life of 1.48 ± 0.53 h (Fan et al., 2015). Another study used an equilibrium dialysis approach to determine acacetin's protein binding in human plasma.The results revealed that acacetin binds extensively to human plasma protein with a recovery range of 91.5-95.6% in a concentration-independent manner (Kim et al., 2016) . In another study, the extract of Cirsium japonicum DC was administered orally at a dose of 6 mL/kg to male Sprague-Dawley rats, and acacetin concentration was determined simultaneously with other flavonoids by LC-MS/MS method. After oral administration, acacetin was absorbed rapidly and reached its maximum concentration (Cmax = 19.02 ± 1.29 ng/mL) after 5 minutes of administration. The half-life of acacetin was observed at 69.17 ± 6.86 minutes (Zhang et al., 2014) .
Recently, dietary acacetin metabolites found in honey were studied in vivo and in vitro. The acacetin metabolites were identified and quantified using UHPLCQ-TOF-MS/MS, an unique analytical method. There were 31 acacetin metabolites identified. Acacetin was metabolized by the oxidation, reduction, hydrolysis, sulfate conjugation, glucuronide conjugation, N-acetylation, loss of CH2 and methylation processes (Yin et al., 2019) . Apparently tilianin is converted to acacetin, glucuronide, and tilianin enabled enterohepatic and enteric recycling by bacterial-glucuronidases hydrolyzing acacetin glucuronide. However, gluconolactone (20 mM) alone or in conjunction with saccharolactone (0.1 mM) reduced tilianin absorption, which lowered biliary and enteric excretion of acacetin glucuronide. (Dai et al., 2015) .
Conclusion
It is concluded that acacetin has diverse pharmacological potential and has multiple roles if applied in a range of concentrations or doses. Acacetin has cytoprotective and cytotoxic roles depending upon the concentration and the tissue type where being applied. Acacetin is effective against various microbial diseases, cardiovascular issues, neurological disorders, inflammatory conditions, rheumatoid arthritis, antipyretics, blood glucose levels, anti-cancer potential, ant-aging and antioxidant activities and much more. The mechanism behind acacetin pharmacological actions is variable, i.e., it acts as an inhibitor of enzymes by direct binding at the active sites, stops protein phosphorylation, stops ion channels, and activates intracellular signaling molecules. These properties make acacetin an appealing candidate to be designed and screened as a multipurpose inhibitor against microbes, viruses, various enzymes, and proteins that help in the disease progression.
Author contributions
Research concept and design: Liangliang Yao, Suyou Zhu, Wei Liu; Collection and/or assembly of data: Liangliang Yao, Mingxi Li; Data analysis and interpretation: Liangliang Yao, Suyou Zhu; Writing the article: Liangliang Yao, Suyou Zhu, Wei Liu, Zahid Manzoor, Muhammad Farrukh Nisar, Mingxi Li; Critical revision of the article: Muhammad Farrukh Nisar; Final approval of the article: Mingxi Li.