1. INTRODUCTION
Vitamin C, also known as L-ascorbic acid, is a vital water-soluble nutrient well known for its powerful antioxidant properties and its involvement in numerous biological functions crucial for maintaining human health. It plays a significant role in promoting collagen production, guiding keratinocyte maturation, controlling melanin synthesis, and counteracting oxidative stress—all of which contribute to immune defence and overall antioxidant support (Papaccio et al., 2022; Wang et al., 2018).
However, maintaining vitamin C’s stability in supplemental forms remains a major challenge due to its chemically fragile structure. The γ-lactone ring of ascorbic acid is highly unstable and easily degrades when exposed to environmental factors such as heat, light, oxygen, alkaline conditions, and metal ions like copper and iron (Pullar et al., 2017; Yin et al., 2022). When exposed to ultraviolet light, it readily breaks down into compounds like dehydroascorbic acid and 2,3-diketogulonic acid, which diminishes its biological efficacy (Ahmad et al., 2011; Ho et al., 2023).
At physiological doses (30–100 mg/day), vitamin C is primarily used for maintaining antioxidant defences, collagen synthesis, and wound healing (Hemilä, 2017). A 1000 mg/day intake of vitamin C increased IL-6 and IL-10 levels, suggesting a focused improvement in immune signaling (Żychowska M, et. al., 2022). However, therapeutic effects- such as antiviral activity, anti-inflammatory responses, or cytotoxic effects on tumor cells- are often associated with much higher tissue or plasma concentrations than those achievable via conventional oral dosing. These effects may require pharmacologic plasma levels, often exceeding 1000 µmol/L, which can only be reliably achieved via intravenous administration or improved delivery systems such as liposomes (Carr and Cook, 2018; Padayatty et al., 2006).
Additionally, the absorption efficiency of conventional oral Vitamin C supplements is limited. Absorption primarily occurs through sodium-dependent vitamin C transporters (SVCTs), which become saturated at moderate doses. This means that increasing the oral dosage beyond 200–400 mg does not proportionally raise plasma levels, as the excess is rapidly excreted in urine. Once the dose exceeds 400 mg, plasma concentrations begin to plateau due to saturation of the intestinal SVCT1 and SVCT2. (Carr and Maggini, 2017; Levine et al., 2011; Lykkesfeldt and Tveden-Nyborg, 2019; Padayatty et al., 2016). Such saturation effects restrict the use of traditional formulations in clinical settings where higher systemic concentrations may be needed.
Liposomal formulations offer a novel means of mitigating these stability-related challenges. Liposomes are nanoscopic vesicles with phospholipid bilayers that mimic the structure of cell membranes, offering both hydrophilic and lipophilic properties (Ko et al., 2023; Liu et al., 2020). This resemblance enhances cellular uptake through alternative routes such as passive diffusion and endocytosis, potentially avoiding SVCT saturation (Pandya et al., 2021). Moreover, the lipid bilayer safeguards the encapsulated Vitamin C from environmental degradation and limits hepatic first-pass metabolism, which may lead to improved bioavailability over nonliposomal forms (Lee, 2020; Shade, 2016).
To empirically evaluate the potential advantages, a randomized, open-label, crossover clinical trial was conducted comparing liposomal and nonliposomal vitamin C in healthy volunteers. The primary goal was to assess and compare pharmacokinetic parameters such as plasma levels, distribution, clearance, and elimination following a single dose of each formulation. Secondary outcomes focused on evaluating safety and tolerability. The results aim to provide evidence-based insights for optimizing Vitamin C supplementation, particularly in contexts requiring enhanced bioavailability and therapeutic efficacy.
2. MATERIALS AND METHODS
2.1. Recruitment and randomization
The selection process adhered to ethical protocols, with all subjects or their legal representatives signing a written informed consent form before taking part in the study. A detailed screening procedure ensured that only eligible individuals were included in the study, while those who did not meet the inclusion criteria or presented exclusionary factors were excluded. Eligible participants were healthy men and nonpregnant women between 18 and 45 years of age, with a body mass index (BMI) ranging from 18.5 to 24. All candidates had to be free of underlying medical conditions, as verified through medical history, physical examination, ECG, chest X-ray (posteroanterior view), and laboratory assessments performed within 7 days before the study began.
Individuals were excluded if they had a history of, or currently suffered from, major cardiovascular, respiratory, liver, neurological, or psychiatric illnesses; abnormal blood pressure; pulse rates below 50 or above 100 beats per minute; difficulties with blood donation or swallowing; or if they had participated in another clinical trial within the previous three months. Participants were also excluded if they had any known allergies to vitamin C products, heparin food, or other drugs; or if they had taken over-the-counter or prescription medications, including enzyme-modifying drugs, herbal products, or vitamin supplements, within 14 days prior to the study, as these may interfere with drug metabolism.
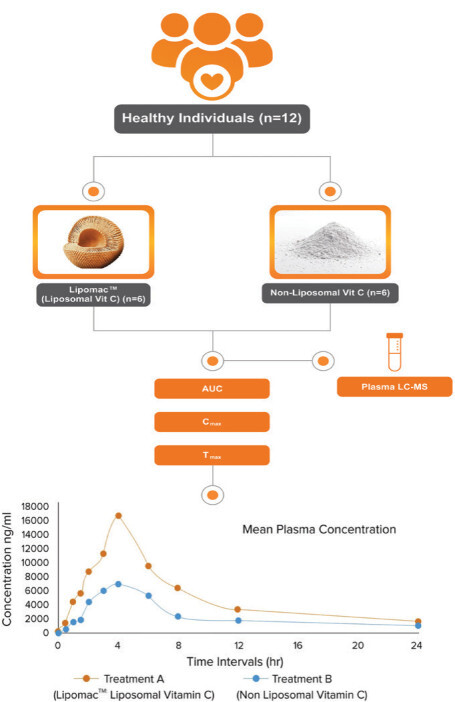
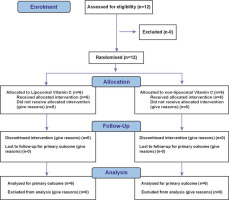
The study CONSORT diagram is illustrated in Figure 1. Demographic details of participants are given in Table 1. The study was carried out at Medstar Specialty Hospital, Bengaluru, Karnataka, India. The study received ethical approval under the IEC reference number RRS/CL/BA/LP1/2024. This study is registered on the Clinical Trial Registry of India (Registration no. CTRI/2024/04/066202). Twelve participants were screened for eligibility and were randomized into two groups. One group received liposomal vitamin C formulation while another group received nonliposomal vitamin C.
2.2. The supplementation
The investigational product for this study was liposomal vitamin C (LipomacTM), and the control product was nonliposomal vitamin C.
2.3. Outcome measures
The main aim of this research was to assess and compare the bioavailability of vitamin C in both liposomal and conventional (nonliposomal) formulations. Additionally, the study sought to evaluate the safety profile and tolerability following a single dose of each vitamin C formulation.
2.4. Safety analysis
Safety parameters for this study included the vitals, adverse events, and laboratory safety parameters were compared from baseline to the final visit of the subjects.
2.5. Statistical analysis
All statistical analyses were performed using SAS software version 9.4. For quantitative variables, descriptive statistics such as sample size (N), mean, standard deviation, median, minimum, and maximum values were calculated. A paired t-test was applied to assess changes from baseline across treatment groups, while ANOVA was used to evaluate differences between the test and comparator groups.
3. RESULTS
3.1. Demographic and baseline characteristics
Vital parameters including temperature, heart rate, respiratory rate, and blood pressure were recorded at the screening visit and subsequent visits. There was no statistically significant variation in vital parameters during the study visits.
3.2. Physical examination
All the study subjects were monitored for any adverse effects on various systems by physical examination during the study visits, and there was no any difference in these parameters during the study visits.
3.3. Pharmacokinetic parameters
Plasma concentrations of vitamin C were measured at multiple time points over 24 h for both liposomal vitamin C (Treatment A) and nonliposomal vitamin C (Treatment B). Key pharmacokinetic parameters, including maximum concentration (Cmax), time to reach maximum concentration (Tmax), and area under the curve (AUC), were assessed to determine the absorption profile (Tables 2 and 3).
Table 2
Vitamin C concentration in plasma at different time intervals with treatment A (ng/ml).
Table 3
Vitamin C concentration in plasma at different time intervals with treatment B (ng/ml).
Liposomal vitamin C (Treatment A) demonstrated a higher mean Cmax of 16,462.86 ng/mL with a Tmax of 4 hrs and an AUC of 1,22,809 ng•h/L, indicating enhanced absorption and sustained release (Table 4).
Table 4
Pharmacokinetics parameters of vitamin C with liposomal formulation.
Nonliposomal vitamin C (Treatment B), in comparison, had a lower mean Cmax of 6,950 ng/mL, an identical Tmax of 4 hrs, and an AUC of 60,506 ng•h/L, suggesting lower bioavailability (Table 5).
Table 5
Pharmacokinetics parameters of vitamin C with nonliposomal formulation.
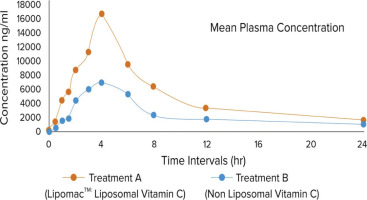
The graphical representation in Figure 2 illustrates the plasma concentration profiles of Treatment A and Treatment B over different time intervals. Liposomal vitamin C exhibited superior pharmacokinetic properties, achieving higher plasma concentrations and a greater overall exposure (AUC) than the nonliposomal formulation. This indicates that liposomal encapsulation enhances the stability and absorption of vitamin C, making it a more effective delivery system. Paired comparisons of treatment groups of vitamin C levels in plasma by parametric test (Independent Samples Test) is given in Table 6, while paired comparisons of treatment groups of vitamin C levels in plasma by nonparametric test (Mann–Whitney Test) is given in Table 7. Results of the test between and within groups on the effects of vitamin C concentration in plasma by parametric test (ANOVA) are given in Table 8. Results of the test between and within groups on the effects of vitamin C concentration in plasma by nonparametric test (Kruskal–Wallis Test) are given in Table 9.
Table 6
Paired comparisons of treatment groups of vitamin C levels in plasma by the parametric test (Independent Samples Test).
Table 7
Paired comparisons of treatment groups of vitamin C levels in plasma by nonparametric test (Mann–Whitney Test).
| Test statisticsa | |
|---|---|
| Group | Treatment A − Treatment B |
| Mann–Whitney U | 0.000 |
| Wilcoxon W | 78.000 |
| Z | −4.158 |
| Asymp. Sig. (2-tailed) | 0.000 |
| Exact Sig. [2*(1-tailed Sig.)] | 0.000b |
4. DISCUSSION
A total of 12 participants completed the study, and patient compliance with the investigational product was 100%. There were no dropouts or lost-to-follow-up patients by the end of the study. The demographics of the study subjects include average height, weight, and BMI of enrolled patients measured on screening; baseline visit and follow up are matched. No clinically significant abnormalities were found in either the test or control group, suggesting that the study drug appears to be safe for use. Pharmacokinetic parameters such as Cmax and AUC were studied in this study over the period of 24 h after single dose administration through oral route. The mean Cmax in liposomal vitamin C formulation was 16,462.86 ng/mL, whereas mean Cmax in nonliposomal vitamin C formulation was 6,950.59 ng/mL. Liposomal vitamin C group was found to be statistically significant over nonliposomal group at 4 h (p < 0.0001). The mean AUC in liposomal vitamin C formulations was 1,22,809 ng/H/L and in the nonliposomal group was 60,506 ng/H/L over the period of 24 h (p value < 0.0001). The data indicate that the liposomal vitamin C group exhibited superior pharmacokinetic parameters and significantly enhanced oral bioavailability compared to the nonliposomal formulation. When assessed based on the mean Cmax levels in plasma, the relative oral bioavailability of liposomal vitamin C was 2.36 times higher, reaching 16,462.86 ng/mL, in contrast to 6,950 ng/mL observed with the nonliposomal form.
The improved pharmacokinetic profile observed in this study can be attributed to the liposomal delivery system, which protects vitamin C from degradation in the gastrointestinal tract and facilitates efficient absorption across the intestinal epithelium. Liposomes, being phospholipid-based vesicles, are known for enhancing drug solubility and permeability and promoting sustained release and systemic delivery (Akbarzadeh et al., 2013).
The results obtained from this clinical study were comparable to another study (Gopi and Balakrishnan 2021) Comparison of Cmax values revealed that liposomal vitamin C exhibited 1.77 times greater bioavailability than the nonliposomal formulation, whereas in our study, it was found that Liposomal vitamin C demonstrated a 2.36-fold increase in bioavailability compared to the nonliposomal form, which shows better results compared to that study. These findings highlight the potential of liposomal delivery systems to enhance the absorption and efficacy of nutrients like vitamin C. By improving bioavailability through encapsulation, this approach may lead to greater clinical benefits, more efficient formulations, and better patient compliance.
The novelty of this study is its direct comparison of liposomal and nonliposomal vitamin C under rigorously controlled clinical conditions, using validated pharmacokinetic assessments over a 24-hour period. The crossover design strengthens the reliability of within-subject comparisons and offers robust insights into pharmacokinetic performance. Although the study focused on bioavailability, the results provide a strong foundation for future research aimed at evaluating clinical outcomes. This paves the way for larger, controlled trials to determine whether enhanced bioavailability translates into meaningful therapeutic advantages, especially in conditions requiring antioxidant or immune support.
5. CONCLUSION
This study evaluated the safety and pharmacokinetic parameters of a liposomal vitamin C formulation in comparison to a nonliposomal supplement. The ingestion of liposomal vitamin C, as compared to the nonliposomal supplement, produces a differential effect on blood vitamin C concentrations, volume of distribution, clearance rates, and elimination patterns. The liposomal vitamin C demonstrated significantly higher Cmax and AUC values compared to the nonliposomal form, with a p-value of 0.0001, indicating statistical significance. The relative bioavailability was 2.36 times greater in the liposomal group. Based on this, the null hypothesis was rejected, confirming a significant difference in bioavailability between the two formulations. The results demonstrate that the liposomal formulation serves as a more efficient delivery system and shows promise for enhanced therapeutic efficacy. Significant differences in plasma concentration, volume of distribution, clearance, and elimination rates further emphasize its potential for improving vitamin C bioavailability. Collectively, the pharmacokinetic advantages observed support the use of liposomal technology as a superior approach to vitamin C supplementation. This study lays a strong foundation for future large-scale, controlled trials to evaluate its clinical benefits and broader therapeutic applications.