Introduction
Cowpea is one of the legumes cultivated throughout the world for their nutritional, agricultural and economic value and as a source of food for humans and animals. Economic agriculture of legumes is based on the efficient symbiotic nitrogen fixation in their nodules, which depends on the rhizobial populations of the soil (Postgate, 1972). The undesirable effects of inorganic fertilizers on the environment have stimulated studies on new natural resources of fertilizers, bio-stimulants and soil amendments. Seaweed biomass represents a substitute for conventional inorganic fertilizers. The favourable effects of SE applications on seed germination and establishment, enhanced crop response and yield have been investigated in cowpea (Sivasankari, Venkatesalu, Anantharaj, & Chandrasekaran, 2006), soybean (Rathore et al., 2009) and maize (Rengasamy et al., 2015a, b) and extensively reviewed byKhan et al. (2009) and Craigie (2011). The beneficial outcome of SE application on crop plants is due to various components that may work synergistically at different concentrations. Moreover, SE is readily biodegradable, free from toxins, non-polluting and non-hazardous. Plant growth regulators, such as cytokinins (Stirk, Novák, Strnad, & Staden, 2003), auxins (Stirk et al., 2004), gibberellins (Stirk, Tarkowská, Turečová, Strnad, & Staden, 2014), betaines (Blunden, 1991; Whapham, Blunden, Jenkins, & Hankins, 1993), micro and macronutrients, relatively low molecular weight compounds, such as polyamines and brassinosteroids (Stirk et al., 2004; Stirk et al., 2014) and major components polysaccharides (Gonza´lez, Castro, Vera, & Moenne, 2013), polyphenols (Rengasamy et al., 2015b) essential for the plant growth and development, have been reported from seaweeds.
Our literature survey could ascertain that recent works on the effects of SE on beneficial soil microbes. The beneficial effect of SE on Rhizobium (CP-1 and MB-1 of cowpea miscellaneous group) in culture has been studied (Thevanathan, Dinamani, Anjanadutta, Pari, & Bhavani, 2005).Ishii et al. (2000) observed that alginate oligosaccharides, obtained from brown seaweed, significantly enhanced AM fungi' hyphal growth and prompted their establishment on trifoliate orange seedlings roots. Extracts of various green, red and brown seaweeds could be used as an AM fungi growth enhancer (Kuwada et al., 1999, 2000, 2006). So far, no study deals with the synergetic effect of seaweed extract and Rhizobium on crop plants, and the present study was initiated to fill the lacuna. Therefore the objective of this study was to determine the effects of a seaweed extract alone or with the application of Rhizobium biofertilizer on the growth, yield and biochemical parameters of Vigna unguiculata.
Materials and methods
Collection of seaweeds
In June 2014, the seaweed [Sargassum wightii Greville (Phaeophyceae)] was taken from the Rameswaram shore (9° 25' N and 79° 15' E), Tamil Nadu, India. The fronds were handpicked and cleaned thoroughly by washing with seawater followed by freshwater. They were then transported to the laboratory using an icebox.
Preparation of seaweed extract
SE was prepared using the approach of (Rao, 1990). Seaweed that had been washed and cleaned was shade-dried for five days and then oven-dried at 60-65 oC for 24 hours before being ground coarsely in a mixer-grinder. The powder was soaked in distilled water at a 1: 20 (w/v) ratio and autoclaved for 60 minutes at 121 oC, 20 pressure. The hot seaweed extract is filtered and cooled with Whatman No.1 filter paper. The filtrate was then centrifuged for 30 minutes at 1500 x g at 4 oC. It was then kept at 60 °C for 48 hours.
Selection of crop plant and Rhizobium biofertilizer
Vigna unguiculata (L.) Walp Var. pusa 151 (family Fabaceae), commonly known as cowpea was study plant. Seeds were obtained from Tamil Nadu's Regional Pulses Research Station in Vamban, Pudukottai District. Healthy seeds were separated and stored until further experiments. The Rhizobium (Pon-rhizobium) biofertilizer was obtained from Pasic Biofertilizers, Puducherry (India).
Experimental procedures and biochemical analysis
The seeds were immersed in different concentrations of SE (0.5, 1, 1.5 , and 2 %) for 24 hours; the control seeds were steeped in distilled water for the same amount of time. The experiment was carried out in earthen pots (30 cm in diameter and 40 cm in height), which were filled with a mixture of sterilised garden soil and sand in a 2:1 (volume/volume) proportion. A total of 10 seeds were sown in each pot, with three pots per concentration remaining after the seeds had been soaked overnight. Two parallel sets were prepared for the two treatments, one set contained the SE-soaked seeds, and the other set included SE-soaked seeds inoculated with Rhizobium biofertilizer (applied as layering on the soil surface). The pots were arranged in three separate units for sampling on different days (viz., 15th, 30th and 45th days) to ensure uniform environmental conditions for the growth of the plants. Pots were watered once every two days. A respective concentration of SE (10 ml) was applied to the rhizosphere at 10-day intervals. A random sample of plants from each treatment was chosen for analysis. The length of shoots and roots, the fresh weight of shoots and roots, the number of lateral roots, the total leaf area, and other characteristics are measured. Plant pigments (Arnon, 1949; Kirk & Allen, 1965) were measured at 15th, 30th and 45th days after the emergence of the 1st set of leaves. Total protein (Lowry, Rosenbrough, Farr, & Randall, 1951), lipids (Folch & Standby, 1956), sugars (Willis & Yemm, 1954) and amino acids (Moore & Stein, 1948) and yield parameters were recorded on the 45th day.
Physico-chemical analyses of SE
The SE color was observed visually, and its pH was measured using an Elico pH meter. Magnesium, iron, zinc, boron, copper, cobalt, calcium, sodium, potassium, manganese, chloride, sulfate, and nitrate contents were analyzed in the extracts following previous method (Apha, Awaa, & Wpcf, 1995), using ICP - OES (Perkin Elmer Mayer, Optima 2100 DV, and Flame Photometer – Systronics.
Results and Discussion
The Sargassum wightii extract was brown, and its pH was 6.6. The elemental compositions for micro and macro elements are presented in Table 1.
Table 1
Elemental composition of Sargassum wightii extract (g kg-1)
Element | Concentration |
Copper | 0.8 |
Zinc | 1.1 |
Boron | 3.8 |
Iron | 6.9 |
Sulfate | 39.50 |
Phosphorus | 54.20 |
Nitrate | 114.10 |
Magnesium | 167.1 |
Calcium | 170.0 |
Potassium | 310.0 |
Sodium | 490.0 |
Chloride | 1180.0 |
Seed germination was 100% at 1% and 1.5% SE with or without biofertilizer and was 90% in other concentrations (0.5% and 2%) and control. The SE alone and with biofertilizer at 1% increased the growth parameters than control. (Figure 1).
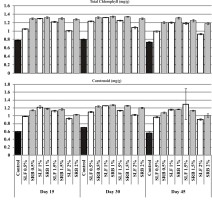
The highest total chlorophyll and carotenoids concentrations were recorded in both the treatments (SE and SE+RB) at 1% seaweed extract on the 30th day (Figure 2).
Figure 2
The effect of SE and Rhizobium biofertilizer treatments on photosynthetic pigments (mg/g) of V.unguiculata
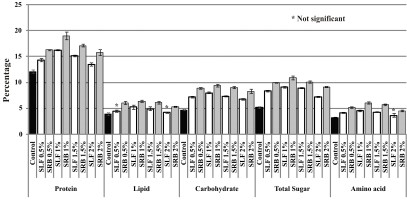
Application of 1% SE alone or rhizobium increased protein, lipids, sugars, and amino acid content significantly (p≤ 0.05) over the control (Figure 3).
Figure 3
The effect of SE and Rhizobium biofertilizer treatment on biochemical parameters (µg/g) of V. unguiculata
The highest number of pods, number of seeds per pod and seed weight were recorded at 1 % seaweed extract in both the treatments (Figure 4).
The yield was significantly increased in all concentrations of seaweed extract compared with the control.
Seed germination
The seeds of V. unguiculata treated with lower concentrations (1% and 1.5%) of SE with or without biofertilizer application showed the highest germination rate. Similar observations were made in V. sinensis (Sivasankari et al., 2006), vegetables and fruit crops (Hong, Hien, & Son, 2007), Triticum aestivum (Kumar & Sahoo, 2011) and V. mungo (Kalaivanan & Venkatesalu, 2012). The observed plant stimulant activity of seaweed extract was much better than previous reports.
Seedling growth
The seeds treated with the lower concentrations of SE, i.e., 1% and 1.5%, with or without rhizobium, resulted in increased dry weight of shoot and roots, lateral roots and leaf area relative to higher SE concentrations and the control (Figure 1). Vasantharaja et al. (2019) reported that treatment with a lower concentration (3%) of S. swartzii extract significantly improved the growth characteristics of V.unguiculata, which is consistent with the current study. Similarly,Battacharyya, Babgohari, Rathor, and Prithiviraj (2015) also find seaweed extract at lower concentrations enhanced the growth of many plants. In support of the findings of the present study, seaweed extract treatment increased the plant vegetative growth, leaf area, plant biomass and total volume of the root system (Elansary, Skalicka-Woźniak, & King, 2016; Hernández-Herrera, Santacruz-Ruvalcaba, Ruiz-López, Norrie, & Hernández-Carmona, 2014; Lola-Luz, Hennequart, & Gaffney, 2013; Mancuso, Azzarello, Mugnai, & Briand, 2006). In addition to growth hormones, nutrients like nitrogen, phosphorus and potash are essential for cell division, cell enlargement and plant growth. The enhanced growth of V. unigiculata may be attributable to such essential elements as nitrogen, phosphorous and potassium in the seaweed extract. Furthermore, calcium and sulfur stimulate the microbial (Rhizobium) activity and formation of nodules, respectively, which contribute to plant growth. These observations supportRathore et al. (2009) 's statement that the micro and macronutrients in the seaweed extract promote plant growth (Singh et al., 2016). Similar increased seedling growth from the application of S. wightii extract have been reported for V. sinensis (Sivasankari et al., 2006), Gossypium hirsutum (Ragavendra, Lokesh, & Prakash, 2007) and Triticum aestivum (Kumar et al., 2011). Researchers found that Ecklonia maxima eckol enhanced the growth of maize seedlings, according to (Rengasamy, Kulkarni, Stirk, & Staden, 2015; Rengasamy, Kulkarni, Stirk, & Staden, 2015) .
Biochemical contents
Photosynthesis is the process by which plants capture solar energy and store nutrients. The amount of plant productivity is determined directly by the photosynthetic ability of leaves. Seaweed liquid fertilizer applied at 1% with or without the Rhizobium biofertilizer produced higher amounts of pigments, such as chlorophyll and carotenoid, in V. unigiculata compared with higher concentrations of SE and control (Figure 2). The chlorophyll content of leaves was raised by the application of seaweed extract in similar investigations (Whapham et al., 1993; Spinelli et al., 2010; Krainc et al., 2012; Jannin et al., 2013; Yao et al., 2020) (Jannin et al., 2013; Spinelli, Fiori, Noferini, Sprocatti, & Costa, 2010; Whapham et al., 1993; Yao, Wang, Chen, Zhang, & Ma, 2020). The elements, such as nitrogen, copper, iron, magnesium, sulfur, zinc and manganese, are involved in the photosynthesis and production of chlorophyll. At lower concentrations of SE, the seaweed extract had appropriate levels of nutrients, which may have contributed to the rise in pigment content (Clárk, 1983).
Similarly, Paul and Nongkynrih (1996) reported that the catalytic activities, synthesis and maintenance of chlorophyll in seaweed extract-treated plants were attributable to the presence of iron, copper and magnesium in the SE concentrates. This could be attributable to the presence of essential minerals, such as iron, nickel, copper, and magnesium, in the seaweed extract (Fanero, Cattalini, Bertaggia, & Albergoni, 1996). The foliar application of seaweed extract significantly stimulated photosynthesis, carbon, nitrogen and sulphur metabolism (Jannin et al., 2013). The improved growth response of crop plants supplemented with seaweed extract is due to the presence of micro and macronutrients (Singh et al., 2016), polysaccharides (Rolland, Moore, & Sheen, 2002), plant growth hormones (Khan et al., 2009).
Seaweed extract applied at 1% with or without the Rhizobium biofertilizer enhanced protein, lipid, sugar and amino acid contents in V. unigiculata relative to other concentrations of SE and the control (Figure 3). The increased biochemical constituents at the lower concentrations of SE might be on account of the absorption, by the seedlings, of most of the necessary elements, such as nitrate, potassium, copper, zinc and boron. These elements are necessary for the synthesis and translocation of various biochemicals and their metabolism. This is supported by the observations of KKannan and Selvan (1990) in Vigna radiata. Tamil Selvan reported similar trends and Kannan (1994) in Vigna mungo treated with Hypnea musciformis extract and bySivasankari et al. (2006) in V. sinensis and Vasantharaja et al. (2019) in V.unguiculata treated with Sargassum extract.
Yield
The favourable effects on yield parameters at the lower concentration (1%) of SE alone and with Rhizobium (Figure 4). Similarly, encouraging effects on the yield of ‘Thompson seedless’ grape (Vitis vinifera L.) (Norrie & Keathley, 2005), Capsicum annuum (Arthur, Stirk, & Staden, 2003), T. aestivum (Kumar et al., 2011), Vigna radiata and Vigna mungo (Pramanick, Brahmachari, & Ghosh, 2013; Pramanick, Brahmachari, Ghosh, & Zodape, 2016) and Solanum lycopersicum (Yao et al., 2020) have been reported with the use of seaweed extract. The presence of hormones, particularly cytokinins, in the extracts of seaweed-treated plants is thought to be associated to increased production (Featonby-Smith & Staden, 1983; Featonby-Smith & Staden, 1983; Featonby-Smith, 1984). According to Singh et al. (2016), micro-and macronutrients in seaweed extract may activate signaling pathways, synergising plant physiological processes, thereby increasing crop productivity.
The SE application alone at 1% and with Rhizobium showed the most prominent effects in all the tested parameters (vegetative growth, biochemical and yield), whereas 0.5% and 1.5% seaweed extract in both treatments produced more or less similar effects but lower than those at the 1% concentration of tested parameters. The effects were further reduced at 2% compared with the other three concentrations, but they remained higher than the controls. Among the two treatments, the effect was comparatively higher in SE with Rhizobium treatment in all the tested parameters than that of SE treatment alone. According toEyras, Rostagno, and Defoss´e (1998) and Moore (2004) , this may be due to the application of SE, which may have increased soil aeration and capillary activity of soil pores, which stimulated plant root development and soil microbial activity. The results revealed that the difference in the effects between the two treatments and three different sampling days was highly significant for all the growth and biochemical parameters observed.
Conclusions
We concluded that the seed-soaking treatments using the extract from Sargassum wightii alone and in combination with Rhizobium biofertilizer increased biochemical parameters, which resulted in improved germination, growth, pigment content and yield characteristics of Vigna unguiculata. The growth-promoting properties of the seaweed extract and the cumulative effect with Rhizobium were confirmed. Seed treatment of SE with Rhizobium biofertilizer was more effective than the SE alone in enhancing cowpea's growth, yield, and biochemicals. These findings suggest that combining seaweed extract with rhizobium to promote cowpea growth and yield in sustainable agricultural systems could be a viable option. Furthermore, biological-based alternatives reduce the use of synthetic fertilizer and its negative impacts on the biosphere.