Introduction
Asthma is the most prevalent disease that is characterised by airway inflammation. It can be triggered by several issues, including histamine, chemicals, medicines, respiratory infections, dust, cold air, exercise, emotions, and allergies (NHBLI, 2007). A considerable socioeconomic burden and high death rate are associated with the disease of asthma. Asthma is projected to impact 34.3 million Indians, or 13.09% of the global burden of disease, according to the World Burden of Disease Report in 2019. Additionally, It also stated that asthma was the cause of 13.2 thousand fatalities in India. It is responsible for 27.9% of DALYs (Disability-Adjusted Life Years) among Indians. India has a three times higher overall death rate and more than twice as many DALYs from asthma than the worldwide incidence of the illness (Singh, Salvi, & Mangal, 2022).
To combat the progression of asthma, in many settings, treatment of acute attacks and management of its chronic forms have been tackled by synthetic medicine. These medications typically reduce or eliminate symptoms, but they also have a range of side effects, particularly when taken frequently and in high dosages (Papi, Blasi, & Canonica, 2020; Sim, Lee, & Lee, 2014). Considering this, researchers are more likely to choose herbal medicines that are derived from plants because they will be more effective, cost-effective, and toxic-free (Khan & M, 2023). Herbal therapy, which is a branch of complementary and alternative medicine, has recently grown in popularity as a cough and asthma treatment. The affordability of herbal medications and the lower incidence of negative effects contribute to their expanding uses by a large population of the world (Topaz & M, 2013).
Bacopa monnieri is a key ingredient in many Ayurvedic herbal preparations and is used in Indian medicine as a nootropic to enhance cognition and memory and also utilises to treat various diseases and disorders (Nemetchek, Stierle, & Stierle, 2017). In this study, we utilized, B. monnieri bioactive compound known as stigmasterol-enriched extract (STIG) has long been utilised in Indian herbal therapy to treat numerous diseases and ailments, such as diabetes, depression, CVD, inflammation, and others (Ibrahim, Parveen, & Zahiruddin, 2022; Jeyasri, Muthuramalingam, & M, 2022).
There is still a lack of information regarding how specific active components of B. monnieri interact with biological proteins, genes, and cells. Therefore, it is necessary to conduct an association analysis of Bacopa monneiri's biological characteristics with respect to probable targets and their possible mechanisms (Jeyasri et al., 2022; Rajan, Preethi, & Singh, 2015). Network pharmacology, which integrates systems medicine analysis with biomedical big data, has recently become an interesting new area of drug development. The network links and interactions between the active ingredients in conventional pharmaceuticals and their target proteins can be built using biomedical big data, thereby exposing the mechanism of therapeutic action (Noor, Qamar, & Ashfaq, 2022). Thus, in addition to experimental research and network pharmacology have contributed to the development of novel therapeutic drugs.
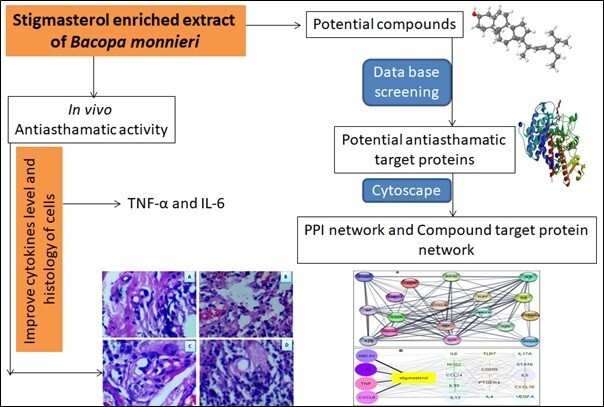
In the current study, network pharmacology and experimental research were combined to assess the potential of stigmasterol-enriched extract (STIG), a bioactive component of B. monnieri. In Figure 1, the study workflow is displayed.
Methodology
Preparation of stigmasterol-enriched extract (STIG)
A distillation flask was filled with B. monnieri and the soxhlet extraction was performed for six hours according to the defined protocol (Poulose, Sajayan, & Ravindran, 2021). After cooling, an equal amount of petroleum ether and water (1:1) was added to separate the extract. The separated layers were collected, and the fractions that contained lipids were combined with 1 M ethanolic KOH and agitated for an extended period. Distilled water was used to thin the mixture, and three parts of diethyl ether were used to extract it. The extract had been dried further over deactivated alumina and anhydrous sodium sulfate. The Salkowski test verified the presence of phytosterols. A rotary evaporator was then used to concentrate the extracted organic phase until it was completely dry.
Characterization of stigmasterol-enriched extract (STIG)
A precoated silica gel aluminium plate with a 200 mm thickness a Camag Linomat V sample applicator and a 6 mm sample wide band were spotted on the plate. The temperature and relative humidity for the experiment were set at 25°C±2°C and 40%, respectively. The densitometer was a CAMAG TLC Scanner 3 linked to win-CATS software, the developing chamber was a 20 × 10 cm glass twin trough chamber, and the syringe was 100 µL (from 2.5 microns). The composition of the mobile phase was ethanol: chloroform (9.8:0.2). TLC plates were dried in an air current once the sample had been applied completely. Further, densitometric scanning was performed at 490 nm on the Camag TLC scanner III (Saluja & K, 2017).
Animals
Healy guinea pigs of either sex were utilised in the experiment. The animals were acquired from an animal house (Approval no. GLAIPR/CPCSEA/IAEC/2022/R7). Every animal was maintained in a cage, and the males and females were kept apart for the first two weeks of acclimatization. The rodents were exposed to the natural light or dark cycle, room temperature, and free access to dry mouse pellets and tap water. They were treated in accordance with accepted practices for working with lab animals.
OVA sensitization and drug administration
The animals (n = 6) were allocated at random to one of four groups: Group 1; considered as the normal control group (NC), Group 2; OVA-I (OVA-induced challenge), Group 3; (STIG) served as the experimental group (OVA-induced challenge and Stigmasterol enriched extract (STIG; 100 mg/kg b.w. treatment), and Group 4 (DEXA) served as the positive control (OVA-induced challenge and dexamethasone at 3 mg/kg b.w. treatment). All the animals, except the NC, were sensitised with intraperitoneal (i.p.) injections of OVA and aluminium hydroxide on days 0 and 8, respectively. Except for the control group, all the animals were exposed to 1% OVA aerosol in saline for 20 minutes every day for 14 days using an ultrasonic nebulizer attached to their cages. Group 3 and 4 animals were given oral STIG 100 mg/kg b.w. and dexamethasone at a dose of 3 mg/kg b.w. one hour following an OVA challenge on day 15 and every day for 14 days, respectively. On day 29, all the animals were sacrificed and collect blood sample biochemical analysis. In addition, a portion of the lung tissue was preserved in 10% neutral buffered formalin for histological analysis.
Haematological estimation
The level of platelets (PLT). red blood cells (RBC) and white blood cells (WBC) were estimated in the blood serum according to the defined protocol (Zn & Alruhaimi, 2020). The quantification of haematological parameters was done using a hemocytometer.
Biochemical analysis
The level of cytokines (IL-4 and TNF-α) in the blood was measured using commercially available ELISA kits in accordance with the manufacturer's recommendations. Further, according to the described protocol, the activity of the antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH) and the oxidative stress indicators malondialdehyde (MDA). Antioxidant activity was performed according to the cited articles (Agrawal, Abdullah, Jha, & PM, 2022).
Histopathological analysis
Hematoxylin and eosin (H&E) staining was applied after lung tissues treated with 10% formalin were allowed to air dry with a gradient of ethanol and then fixed in paraffin. A 40x light microscope was used for both examination and photography of the specimens.
Network pharmacology
GO and KEGG enrichment analysis of target proteins
The biological process and KEGG pathway enrichment of proteins involved in the protein-protein interaction (PPI) network were analyzed using ShinyGO v0.741 to comprehend the function of target proteins that interact with stigmasterol, an active chemical found in B. monnieri.
Results
HPTLC fingerprint
Chromatographic analysis of fingerprints has shown to be a suitable and useful technique for determining the species' authenticity and quality of traditional medicine. The standard stigmasterol and the stigmasterol-enriched extract (STIG) of B. monnieri were separated on pre-coated silica gel F254 HPTLC plates with mobile phase chloroform: ethanol (9.8:0.2 v/v) for fingerprint pattern analysis. The Figure 1 displays the Rf value of stigmasterol (Rf=0.45) and enhanced extract chromatograms of B. monnieri.
The effect of STIG on the haematological parameters
The RBCs, WBCS, and PLT numbers were identified in the treated groups. Significant alterations of RBCs, WBCS, and PLT levels were observed in OVA-challenged groups as compared to the healthy control guinea pigs. After treatment with stigmasterol-enriched extract (STIG), the alteration was ameliorated towards normal. The same observation was also found in standard dexamethasone. The overall results are depicted in Table 1.
Table 1
Effect of STIG on haematological parameters in OVA-induced asthmatic guinea pigs
Effect of STIG on the levels of antioxidant enzymes and MDA
In comparison to the healthy control guinea pigs, the lungs of the OVA-challenged guinea pigs had significantly lower levels of both enzymatic and non-enzymatic antioxidants, such as GSH, CAT, and SOD, indicating an oxidative stress state. When compared to the normal control guinea pigs, malondialdehyde (MDA) levels in OVA-induced guinea pigs were considerably higher (Table 2). By increasing the expression of GSH, CAT, and SOD and lowering the expression of MDA in comparison to OVA-induced guinea pigs, treatment with stigmasterol in asthmatic guinea pigs dramatically reversed the effects of OVA-induced lung injury.
Table 2
Effect of STIG on oxidative stress markers in OVA-induced asthmatic Guinea pigs
Effect of STIG on the expression of inflammatory cytokines
In the OVA-induced guinea pigs, inflammatory cytokines like TNF-α and IL10 were estimated and found higher as compared to the NC group. As shown in Table 3, stigmasterol-enriched extract decreases the level of the inflammatory cytokines in the OVA-challenged group. In comparison to the OVA-induced group, the positive control group also showed a reduced level of inflammatory cytokines.
Histopathological examination
The lung tissue of the NC group of guinea pigs displayed an optimal shape with an organised alveolar space and a thin-lined bronchial wall. Massive inflammatory alterations, altered alveolar space, oedema, intra-bronchial wall and thickening wall were all visible in the OVA-challenged lung sections. Stigmasterol-enriched extract treatment reduced inflammatory cell infiltration, thin intrabronchial walls, and organised alveolar space in asthmatic guinea pigs produced by OVA (Figure 2). The lung morphology of the positive control guinea pigs ats recovered similarly.
GO biological and KEGG analysis of the genes
The biological process studies showed that these targets were engaged in various signalling pathways including neurogenesis, which was particularly significant. Figure 3A and B, represents the different signalling pathways connected to asthma and KEGG enrichment signalling pathways (Figure 3). Therefore, in the current study, we hypothesised that stigmasterol-enriched extract might affect ABAC1, IL10, TNF-α, and CXCLB to ameliorate asthma. Hence, we can conclude that stigmasterol-enriched extract may ameliorate the occurrence of asthma.
PPI network of targeted genes and understanding the action mechanisms of stigmasterol-enriched extract
The PPI network was created after the Network analyser was utilized to analyse the chosen probable gene targets (Figure 4A). The figure demonstrates the close relationships between genes. Additionally, we have chosen the biopotential genes (ABAC1, IL10, TNF-α, and CXCLB) within the same PPI network in order to uncover the mechanism of action of stigmasterol-enriched extract found in B. monnieri. As a result, we built a global perspective of the compound-target-pathway network to better comprehend how stigmasterol-enriched extract works at the molecular level to manage asthma (Figure 4B). The network shown in Figure 4 approaches to manage asthma.
Figure 4
(A) PPI network of asthma-related gene targets of the stigmasterol-enriched extract. Dark color nodes anticipated the most significant genes implicated in the development of asthma. (B) Target enrichment diagram of stigmasterol-enriched extract with TNF-α, IL10, CXCL8, ABCA1 and other proteins related to asthma.

Discussion
The remodelling of lung tissue damage, cellular inflammation, oxidative stress and alteration in inflammatory cytokines associated with allergen-induced asthma is well documented (Zemmouri, Sekiou, & Ammar, 2017; Zn & Alruhaimi, 2020). The results were observed in guinea pig models of our study caused by OVA with significant alteration in haematological parameters, antioxidant levels and inflammatory markers. Moreover, the histological findings, which demonstrated maintained alveolar space and a thin bronchial wall with fewer inflammatory cell infiltrations, supported the results. The OVA-induced animals treated with high doses of stigmasterol-enriched extract (STIG) displayed similar pathological results. The study supports, STIG potential to treat asthma by ameliorating haematological parameters, the level of oxidative stress and inflammation as well as reducing the cell damage and cellular inflammation observed in lung tissues. Our observations are almost similar to previous findings (Antwi, Obiri, & Osafo, 2017; Zhang, Zhang, & Miao, 2023).
Asthma development is known to be significantly influenced by cytokines such as TNF-α and IL-10. Reactive oxygen species and pro-inflammatory cytokines are produced when these cytokines cause WBC activation, as well as alteration, were observed in RBCs and platelets (Kany, Vollrath, & Relja, 2019) (Atawi et al., 2017). Furthermore, by oxidising the membrane phospholipids in a series of processes and producing MDA and other byproducts, the generation of ROS at the cellular site generates oxidative stress, which results in cellular damage. Inflammation is a result of oxidative stress, which is also linked to the emergence of chronic asthma (Pizzino et al., 2007). Pro-inflammatory cytokines also contribute to the disease's progression by damaging the cells near the injury site. The breakdown of membrane phospholipids and the formation of byproducts that act as inflammatory mediators are only two examples of the many elements that influence the activation of the NF-kB pathway. Therefore, reducing oxidative stress may help to stop the development of injury and inflammation (Zhang & J, 2007). Antioxidant enzymes such as GSH, CAT, SOD, and others, play a pivotal role in controlling the production of ROS. The findings showed that OVA-challenged guinea pigs were under oxidative stress because of the low level of antioxidant enzymes and high level of MDA (Gumulec, Raudenska, & Hlavna, 2013). Furthermore, the higher levels of pro-inflammatory cytokines in the asthmatic guinea pigs generated by OVA can be interpreted as evidence that oxidative stress has contributed to the advancement of inflammation. Antioxidant STIG was able to scavenge the generated ROS, halting lipid peroxidation and maintaining antioxidant enzyme levels. This resulted in the influx of pro-inflammatory cytokines into the lung tissues and the suppression of the inflammatory response (Antwi et al., 2017).
Previous studies have demonstrated an association between the generation of ROS and the inflammatory response caused by allergens. It is possible for ROS to interact with intracellular GSH, depleting the antioxidant and bringing on oxidative stress. Similar to these findings, we also showed that ROS levels were significantly elevated while GSH levels were decreased in asthmatic guinea pigs induced by OVA (Mittal, Siddiqui, & Tran, 2014). Increased ROS currently increases the inflammation in the lungs airways by enhancing cytokines associated with Th2 and downregulating Th1 cells. According to the histopathology findings, STIG was able to inhibit the rise in ROS levels, supporting the avoidance of airway inflammation. In the OVA-induced asthmatic guinea pigs, the expression of these inflammatory cytokines was substantially altered, which may be associated with the histological results of the thickening of the intrabronchial wall. Due to the anti-inflammatory properties of STIG, the expression of these cytokines is markedly reduced in the OVA-treated asthmatic guinea pigs (Antwi et al., 2017).
Moreover, network pharmacology is a method for estimating ligand-protein interactions for combining molecular recognition and for rapidly screening databases for precise protein structure and protein-ligand complex prediction for main structure-based drug design. The present investigation of network pharmacology significantly supported the results observed in vivo studies (Obaidullah, Alanazi, & Alsaif, 2022).
As an alternative to the proposed link between asthma, the production of free radical species, and inflammation were ameliorated by STIG. Therefore, it is thought that stigmasterol-enriched extract may lower the likelihood of getting asthma and can be utilised as therapy for managing it from a biological and pharmacological perspective.
Conclusion
Overall, our findings indicate that STIG, a bioactive compound of B. monnieri significantly modulates the level of haematological parameters, oxidative stress and inflammatory cytokines towards normal. It is also supported by the results of network pharmacology. In addition, it maintains the normal structure of lung tissues. Hence, it is affirmed that STIG is therefore a promising alternative medicine for the treatment of asthma. Before STIG is turned into a medicinal product for human use, more investigation is required to confirm its safety and mechanism of action at the molecular level at the clinical stage.