INTRODUCTION
Neolamarckia cadamba has historically been used in the treatment of eye infections, skin illnesses (Umachigi et al., 2007), dyspepsia, gum problems, stomatitis, cough, fever (Pandey & Negi, 2018), anaemia, blood abnormalities, and stomach pain (Dukes, 2007; Khare, 2007) in Indian traditional medicine (Khare, 2007). Different parts of this plant contain various phytochemicals that show an extensive range of biological activities. Phytochemical investigations conducted worldwide reported the presence of various classes of compounds, including triterpenoids, sesquiterpenoids, lignans, steroids, alkaloids, phenolics and flavonoids.
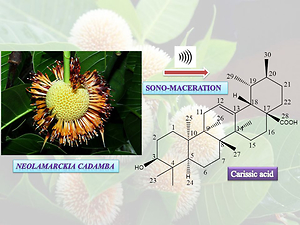
The current research was conducted as part of a quest for bioactive natural compounds from the dried leaves of Neolamarckia cadamba, and it resulted in the discovery of carissic acid, an isomer of ursolic acid. Carissic acid (3 beta-hydroxy-12-taraxasten-28-oic acid) is a triterpenoid with very few articles available for review. Fruits and leaves of Carissa carandas Linn. (F. Apocynaceae) (Naim, Khan, & Nizam, 1988), a perennial (Fartyal & Kumar, 2014) Indian shrub (Singh, Bajpai, & Mishra, 2020), contain this isomer of ursolic acid (Aslam et al., 2011; Siddiqui, Ghani, Ali, Usmani, & Begum, 2003; Singh & Uppal, 2015; Tesfaye & Ravichadran, 2018) and has been recently reported to possess potent in vitro cytotoxic activity against human cancer cell lines (Kour et al., 2020). Carissa carandas, commonly known as Karonda or Christ’s thorn, is widely used to treat scabies, intestinal worms, diarrhoea, fever, and other disorders (Jain, 1991). The plant is also used as an antidote for snakebite (Kirtikar & Basu, 1933). Ethanolic extract of roots has reported histamine-releasing activity (Desai, 1927; Joglekar & Gaitonde, 1970).
The isolated phytochemical has never been found in any Anthocephalus or Neolamarckia species. It was isolated for the first time from this genus. Though carissic acid has been isolated previously (Naim et al., 1988), the information was limited to its isolation, proton NMR and Mass spectra. Nevertheless, in this study, carissic acid isolated from Neolamarckia cadamba leaves have been characterized by spectral techniques including IR, Proton NMR, Carbon NMR, DEPT, HSQC and MS.
MATERIALS AND METHODS
Chemicals, reagents and instruments
Chemicals and reagents employed were extra pure and of analytical grade, purchased from SDF Mumbai. The water used was double distilled. TLC Silica gel 60 F254 Aluminum sheets (Merck, Germany) were used to check the purity of compounds. Column chromatography was carried out using Silica gel (SDF Fine Chemicals) 60-120 mesh in a column of 50 cm length and 3.0 cm diameter.
Ultrasonication was performed using GT SONIC Antech with a power of 100 W and frequency of 40 kHz, equipped with a digital controller displaying cleaning time and temperature. Melting point apparatus (Expo Hi-Tech) was used to record melting point (uncorrected). NMR spectra were documented using a Bruker (300 MHz) NMR spectrometer in DMSO (Internal standard-TMS). IR was recorded using Perkin Elmer FT-IR Spectrometer. The mass spectrum was recorded on UFLC SHIMADZU AB SCIEX 3200 QTRAP LCMS/MS system. Thin-layer chromatography (TLC) was performed on an Aluminum TLC plate, silica gel with fluorescent indicator F254 coating, 5 cm
Plant material
1 kg of fresh Neolamarckia cadamba (Roxb.) Bosser leaves were procured from the University of Mumbai's campus. The Blatter Herbarium at St. Xavier's College in Mumbai validated the plant (Specimen No. 116 of Y A Merchant). The leaves were rinsed twice with distilled water cleaned with a dry muslin cloth to absorb extra moisture before introducing it into a hot air oven for drying. Leaves were dried to obtain a constant weight of 150 grams and finely pulverized with a grinder into a fine dry powder. Fresh leaves had a moisture level of roughly 65 percent. The powder was stored in an air-tight glass container for future use.
Extraction and isolation
Neolamarckia cadamba dried, and powdered leaves were macerated with methanol in a 1:10 ratio at room temperature (r.t.) for three days. Exposure to ultrasonic waves (with frequency: 40KHz and power: 100 W) for up to 30 minutes at regular intervals over eight hours improved the extraction efficiency of the solution (George, Meshram, & Patil, 2021). The solution was filtered using Whatman Grade 1 filter paper. Following filtration, the residue was re-extracted twice. To retain all thermolabile compounds in the extract, the mixed filtrate was evaporated to dryness at r.t. Ethyl acetate and water partition 10.9 grams of crude solid methanol extract. The residue of the crude solid methanol extract undissolved in ethyl acetate was brown in colour and mud-like in appearance. It was dissolved in acetone, filtered and evaporated at room temperature. The acetone extract obtained (1.931 g) was loaded onto a silica gel column for chromatographic separation. Since the extract was highly polar, the column was directly eluted using CHCl3: Ethyl acetate (100%-50:50). At uniform intervals, the eluents were collected, and the separation progress was monitored with the help of TLC (Amzad & Mizanur, 2015; Tanushree, Dey, & Panchanan, 2007). 90% fraction of the above solvent system showed a single spot with minimum impurities in the TLC. It was then evaporated to obtain a pale yellow solid. Pet ether washing of this compound yielded a white amorphous solid showing a single spot in the TLC with Rf value=0.69 (Solvent system= 100% CHCl3). This was the isolated compound (63.0 mg) which was then subjected to spectral characterization followed by structure elucidation and finally- identification.
Structural identification of compound
Table 1
1H and13C NMR chemical shifts (δ, ppm) data of isolated compound (DMSO)
RESULTS AND DISCUSSION
63.0 mg of the compound was isolated from 1.931 grams of crude acetone extract from column chromatography. ESI-MS studies showing molecular ion peak of [M-H]- at m/z= 455.5 corresponds to the molecular formula C30H48O3. The 1H NMR studies displayed five singlet signals at δ0.91 (H-23), 0.67 (H-24), 0.86 (H-25), 0.74 (H-26), 1.04 (H-27) belonging to five tertiary methyl groups. An olefinic proton singlet peak at δ5.12 (H-12) illustrates the ursane skeleton, which was found to correlate with C-12 (δC 124.54) in the HSQC spectrum. A proton doublet at δ2.12 (H-18) specifies a urs-12-ene basic skeleton. The 13C NMR spectral data showed 30 carbon resonances characteristic of pentacyclic triterpenes. They were seven methyl carbons (CH3), nine methylene carbons (CH2), seven methine carbons (CH) and seven quaternary carbons (C). Peaks at δC178.22 (C-28, carboxylic acid carbon), 138.13 (C-13, un-hydrogenated olefinic carbon), and 124.54 (C-12, hydrogenated olefinic carbon) were characteristic to ursane skeleton. The two peaks at 138.13 ppm and 124.54 ppm are due to the carbons at either end of the carbon-carbon double bond. When these spectral analyses were carried out, we found that data obtained were similar to previously isolated Ursolic acid (George et al., 2021) (Figure 3) from Neolamarckia cadamba leaves. Therefore, it was expected to be Ursolic acid (UA). However, differences in a few physical properties paved the way to another natural product. Ursolic acid was off-white, whereas the isolated compound was pure white. UA was soluble in the CHCl3-CH3OH mixture whereas this compound was soluble in DMSO. The melting point of UA was around 276 °C, whereas isolated phytochemicals melted at around 238 °C.
Thus, based on these observations and reported data1 and the predicted NMR values from Chemdraw, this compound was characterised as an isomer of UA and identified as Carissic acid (yield= 3.26 %) (Figure 2).
CONCLUSION
In the present study, an ultrasound-assisted extraction method is described, which led to the isolation of known cytotoxic drug- carissic acid, in a very simple, fast and convenient way with good yield from the leaves of Neolamarckia cadamba. To the best of our knowledge, isolation of carissic acid is reported only from the genus Carissa. This work reports its first-ever isolation from outside this genus. Though this isolation and structure elucidation concerns a known compound, the current study offers primary data for future research work: to study its role in the histamine-releasing activity and to find out whether it can serve as an antidote for snake-bites. Our study adds another source for isolating this cytotoxic phytochemical in bulk at any time of the year. We also confirm sono-maceration as a powerful tool for effectively extracting this phytochemical. The instrumental setup offers much potential in terms of commercialization.