1. INTRODUCTION
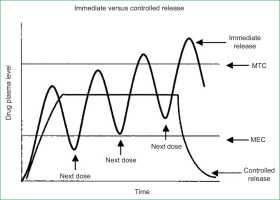
Drug delivery systems (DDS) are methodologies developed to prolong the release time of the drug in the body, sustain its plasma concentration, and control the temporal and spatial localisation of the molecules in vivo through the application of biological and chemical principles (Villanova et al., 2010). Their main objective is to transport the drug to the desired site in the body, release it in a controlled manner, and maintain a constant concentration within the desired therapeutic range in the plasma environment (Pereira et al., 2022). Among drug delivery systems, the syringe is considered to be the first in history (Villanova et al., 2010). According to the story, it was not until the 1960s that hypodermic syringes made of glass, introduced in 1853, began to be replaced by plastic syringes. The drug, in turn, is the substance that is released. It is a chemical substance with a defined constitution and applications as a preventive, curative, or diagnostic agent, encompassing all bioactive compounds with therapeutic intent, from low molecular weight molecules to proteins and genetic material (Rosen & Abribat, 2005). However, conventional modes of drug administration are limited in terms of their action on the body; that is, they are not able to be constantly effective in therapeutic treatments, and can reach high concentrations considered toxic. This leads to the need for multiple doses to maintain therapeutic treatment, depending on the performance of the person administering it within the required periods, as shown in figure 1 (Pereira et al., 2022).
Figure 1
Therapeutic or toxic levels and immediate - versus controlled release dosage form (Shen et al., 2004; Pereira et al., 2022).
There is also some concern about the biocompatibility of materials, since synthetic polymers have been causing greater damage to human and even environmental health, as the United Nations (2018) stated when it warned of the “need to take action against the use of plastic bags as part of the global challenge to reduce ocean pollution” (Farias et al., 2016). On the other hand, non-steroidal anti-inflammatory drugs (NSAIDs) are among the most prescribed drugs worldwide. As it happens, they all have a tendency to cause gastrointestinal adverse effects, which can range from dyspepsia to stomach bleeding (Harirforoosh et al., 2013; Alhammadi et al., 2022). To date, according to data from the Angolan Ministry of Health, “cases of the negative effects of these drugs on the gastric mucosa have risen to frightening levels”, thus creating more problems for the patient rather than solving their condition. In order to resolve such situations, thorough research into the types of materials capable of offering advantages is needed. Thus, biopolymers, being materials derived from biocompatible, versatile, bioavailable, and biodegradable plant molecules, offer advantages as an effective alternative, since these properties reflect the efficacy, selectivity, and safety of the drug (Bodor and Loftsson, 1987). Today, the fusion of polymer science with pharmaceutical science has led to the development of new DDSs in recent decades, mainly in controlled drug release therapies (Oliveira et al., 2006; Pillai & Panchagnula, 2001). Their surface properties (hydrophilicity, lubricity, uniformity, and surface energy) and physical properties (durability, permeability, and degradability) have been important for achieving temporal or spatial control of drug release, enabling controlled-rate delivery from the gastrointestinal tract (Li, 1999; Zhu, 2002; Villanova et al., 2010).
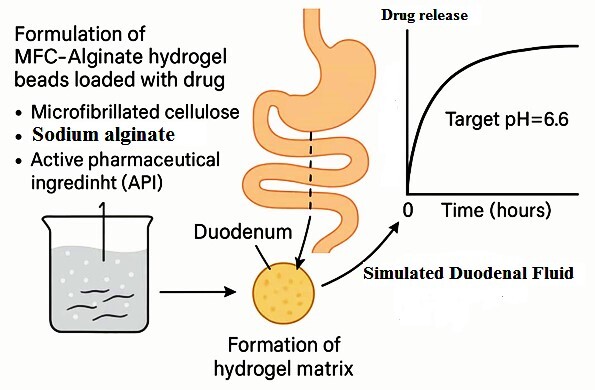
Among the biopolymers chosen is cellulose from Eucalyptus globulus wood, which is economical, abundant, and widely used in countries such as Portugal, Brazil, and Australia for the production of cellulose pulp. It is from these pulps that microfibrillated cellulose (MFC) will be produced for the development of DDS. From this matrix base, on a microscale, therapeutic molecules—namely Diclofenac and Etoricoxib—will be incorporated in order to evaluate their targeting within the gastrointestinal tract (pH 6.6), while avoiding their release at the stomach’s pH (pH 2), due to their harmful effects on the gastric mucosa.
Therefore, the aim of this work is to produce, in the laboratory, DDS based on MFC, in order to prevent the release of Diclofenac at the pH of the stomach (pH 2), directing it in a controlled manner to the duodenum (pH 6.6).
To achieve this objective, the study follows a series of defined procedures. First, MFC will be produced in the laboratory through disintegration and mechanical refining treatment of bleached Eucalyptus kraft pulps, followed by its characterisation. Next, a DDS will be developed using cellulose microfibres (CMF), alginate, CMC, and therapeutic molecules, along with nanofibrillated cellulose previously produced under the guidance of Professor Joana Curto (Curto et al., 2016). The DDS will then be characterised with MFC, and the porosity of the structures will be analysed to optimise the incorporation, transport, and controlled release of therapeutic molecules. Additionally, the study will compare the release kinetics of selected drugs from DDSs containing MFC with those based on unrefined cellulose. Finally, porous polymeric structures will be computationally modelled using a previously validated Matlab simulator (Curto et al., 2011) to investigate the influence of fibre dimensions at the micro and nano scale.
2. MATERIALS AND METHODS
2.1. Materials
The materials used in this work are subdivided into fibres, equipment and chemicals.
The fibrous materials used were bleached Eucalyptus globulus pulp obtained by the unrefined Kraft process, MFC and CMC. The pulps were disintegrated in accordance with ISO 5263.
The equipment includes: an analytical balance; an infrared balance; a scanning electron microscope; an FTIR-ATR spectrophotometer; the ChemSketch programme, version 12.0; a spectrophotometer; and, finally, Microsoft Office Excel 2010.
Note: With the exception of the Scanning Electron Microscope (SEM), which belongs to the Optical Laboratory of the Faculty of Health Sciences, all other equipment is housed in the Fibre Research Laboratory of the Chemistry Department, FibEnTech Unit, at the University of Beira Interior, Portugal.
The chemical products include: sodium alginate, calcium chloride, Diclofenac sodium, Etoricoxib, distilled water, potassium phosphate dibasic, and potassium phosphate monobasic. These products were used to prepare the buffer solutions employed in the release kinetics studies. For incorporation into the DDSs, pure Diclofenac was used to establish the calibration line, along with Diclofenac and Etoricoxib in tablet form (purchased from the pharmacy and approved by INFARMED), both belonging to the NSAID family.
Note: Demineralized water was used in all the experiments in the laboratory.
2.2. Methods
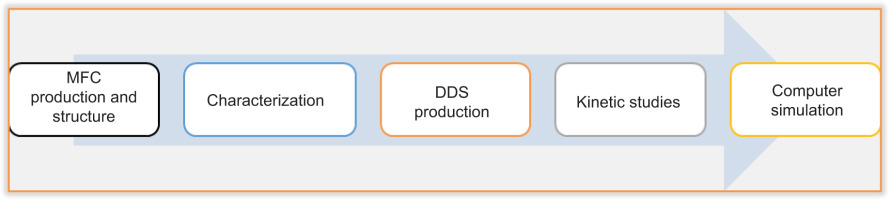
The methodology adopted in this work comprises: the production of MFC and its structure; its characterisation; the production of DDS; kinetic studies; and computer simulation. Figure 2 summarises the methodology adopted.
2.2.2. Disintegration treatment of Eucalyptus globulus fibres
After determining the TMS, a 30 g quantity of dry pulp from a selected batch of kraft pulp was subjected to disintegration (using the disintegrator), so that the fibres could be separated and a fibre suspension, or pulp, could be obtained. The disintegration procedures complied with the ISO 5263 standard. Figure 4 illustrates the main steps and elements used to form the leaf structure.
The elements in Figure 3 show: the disintegrator (a), where the cellulose fibres are placed in suspension; the agitator, still attached to the bucket (b), which prevents the fibres from clumping together; the dynamic former (c), where the sheets are produced; and the formed sheets (d).
2.2.3. Laboratory production of MFC from Eucalyptus globulus Kraft Pulp
MFC was obtained by a mechanical method known as refining, at a consistency of 10%, preceded by disintegration in accordance with standards 5263, 5264, and 5269, using CMC as an additive (at a reference concentration of 0.01%).
Refining is a mechanical process that produces more flexible and homogenised fibres and subsequently structures with smaller pores (Chinga-Carraco, 2011). This process breaks some of the hydrogen bonds between the fibres, causes fibrillation of the cell wall, and can generate fines due to the strong shear forces, which sometimes lead to fibre breakage and a reduction in length. Refining was carried out in the laboratory using a PFI refiner (Figure 4), with 30 grams of pulp at a consistency of 10%, for 9000 revolutions. It was also from this Kraft pulp that the sheet structures were produced, serving as the basis for the production of the DDS, based on the ISO 5269 standard. Figure 4 shows the Eucalyptus globulus Kraft pulp (left) and the microfibrillated cellulose produced (right). Figure 5 presents two structures: unrefined Kraft pulp and refined Kraft pulp.
2.2.4. Characterising the structures produced
After the structures were produced, characterisation followed: biometric characterisation using MORFI to determine fibre size; drainage characterisation using the Schopper-Riegler Grade to assess the difficulty of water drainage from the pulps; morphological characterisation using SEM to quantify the size of the fibres and cellulose pores; surface characterisation of the structures using the contact angle to determine their hydrophobic and hydrophilic properties; analysis of the absorbance of the therapeutic molecules using a spectrophotometer; and, finally, determination of the functional groups and compounds using Fourier Transform Infrared Spectroscopy ATR (FTIR-ATR).
2.2.5. Laboratory production of DDS
The DDS were produced in the laboratory as spheres, following procedures adapted from the literature (Sivakami et al., 2013).
The spheres were prepared by mixing a 2% (w/v) alginate solution with the refined MFC matrix in suspension (20 ml), incorporating the therapeutic molecules (Diclofenac and Etoricoxib) in equal proportions (40 ml), using a homogeniser at 1000 rpm for approximately 30 minutes. Both molecules (Diclofenac and Etoricoxib) belong to the NSAID family, share similar medicinal properties, and were used in their commercial forms. Diclofenac of analytical purity was also used to establish the calibration line.
We used 500 mg in 40 ml (m/v), but it could also be expressed as 0.5 g in 0.4 L (m/v). However, we prefer to work with the units mg and ml, since the drugs are presented in mg. The tablets used are commercial and were obtained from a pharmacy in Portugal (approved by INFARMED, 11.11.2014), with known dosages of 50 mg for Diclofenac sodium and 60 mg for Etoricoxib.
However, in practice, 10 tablets were used for each type of drug:
■ DICLOFENAC:
10 tablets correspond to 500 mg (50 mg × 10 tablets). These tablets were crushed and diluted in 40 ml of water (m/v).
With a known mass (weighed) of 0.5 g, this allowed us to calculate its simple concentration.
■ ETHORICOXIB:
10 tablets correspond to 600 mg (60 mg × 10 tablets). These tablets were crushed and diluted in 40 ml of water (w/v).
With a known mass (weighed) of 0.6 g, this also allowed us to calculate its simple concentration.
However, according to the literature, calculating the drug dosage depends on the “weight” factor of a given patient. Given the limitations of our study (in vitro), we suggest this be addressed in future work (in vivo).
After this process, the prepared mixture was then added drop by drop to a 0.02 M Cacl2 solution using a graduated pipette, in order to obtain approximately 120 spheres. These spheres were left in this solution for 24 hours to harden, forming spherical DDS and allowing the spheres to dry quickly.
Support:
Calcium chloride (Cacl2) is an effective drying agent due to its high capacity to absorb moisture from the air. It is also used in the food industry as a firming agent, which is why it was employed in the preparation of DDS (0.02 M), to facilitate the formation of spherical DDS, enable rapid drying, and allow the spheres to harden (Vijayalakshmi, Gomathi, & Sudha, 2014; Morais, 2017).
Finally, the spheres were filtered, rinsed with distilled water, and drying. Figure 6 shows the drug delivery systems (DDS) produced in the laboratory and their preservation for subsequent analysis.
The drug content was determined based on the chemical formula:
■ “C(x)”: corresponds to the concentration to be determined;
■ “m(x)”: corresponds to the mass of the sample (0.5g = 500mg);
■ “v (x)”: corresponds to the volume of the solution (0.4L = 40ml).
This approach enabled the determination of the concentration of both therapeutic molecules (Diclofenac Sodium and Etoricoxib).
In terms of the drugs incorporated, the following information is added.
2.2.6. Kinetic studies of the release of therapeutic molecules
Once the structures had been produced and characterised, and the drug delivery systems (DDS) with the therapeutic molecules incorporated, they were subjected to a release kinetics study to analyse the absorbance using a UV-Vis spectrophotometer. The studies were carried out in the laboratory, following the procedures described by Vijayalakshmi, Gomathi, & Sudha (2014). The tests involved the spheres (DDS) produced with the incorporation of three therapeutic molecules: one pure (Diclofenac) and two from commercial formulations (Diclofenac and Etoricoxib). The experiments were conducted in triplicate, with a total testing time of 6 hours (three tests, each lasting two hours), at 37°C and approximately 1000 rpm.
[iii] 2*_ 5 – chlorine – 3 (4 - metanossulfonilfenil) -2-(6 – metilpiridini -3 - il) piridina. Sua fórmula molecular é: C18H15ClN2O2S (Fini et al., 2010; Feng et al., 2018).
Three solutions were prepared: an HCl buffer solution to replicate the pH of the stomach (pH 2), a phosphate buffer solution to replicate the pH of the intestine (pH 6.6), and another phosphate buffer solution to replicate the pH of the bloodstream (pH 7.4).
The absorbance of the therapeutic molecules was analysed using a UV-Vis spectrophotometer, with Diclofenac measured at 276 nm and Etoricoxib at 233 nm. The results were presented as time versus percentage release graphs. However, since the studies are in vitro, efforts were made to approximate in vivo conditions as closely as possible.
Figure 7 illustrates the disintegration of the Diclofenac drug from the systems at minutes 20 and 60 of the test, as well as the spectrophotometer used to analyse the absorbances.
2.2.7. 3D representaion of molecules and computer simulation of structures
3D representation of molecules: The 3D structures of the key molecules in the DDSs (Alginate, Cellulose, CMC, Diclofenac, and Etoricoxib) were created to visualise the functional groups of the polymeric materials’ monomers, using ChemSketch version 12.0 software.
Computer simulation of structures and proposal of new DDSs: Structures were produced using a computer simulator implemented and validated in MATLAB (Curto et al., 2011), with assistance from ChemSketch version 12.0, which enabled the modelling of cellulose fibres. The aim was to compare these simulations with laboratory results and predict their behaviour. Microsoft Office Excel 2010 was also used to statistically analyse the research data.
3. RESULTS AND DISCUSSION
3.1. Determining the structural properties of paper sheets
3.1.1. Biometric characterization of Eucalyptus globulus fibres by Morfi
Table 1 presents the results of the biometric analysis of the unrefined MFC pulps and those obtained after the refining process (with the addition of 0.01% CMC).
Table 1
Results of fibre biometric characterization.
| Materiais | Length* | Width | Curving | Mass |
|---|---|---|---|---|
| (Paste m/v) | (mm) | (μm) | (%) | linear (mg/m) |
| Not refined | 0.8 | 19.3 | 7.4 | 0.07 |
| Refined | 0.8 | 18.6 | 7.2 | 0.07 |
It can be seen that the fibres of both samples have similar dimensions in terms of length, width, curvature, and linear mass. The decrease in width observed in the refined pulp fibres is consistent with the loss of part of the fibre wall due to fibrillation. The percentage of fines was also assessed; however, the values obtained require further confirmation.
Note: When considering the biometric analysis of fibres, parameters such as width, twist, bend, and others are relevant. However, length (weighted by length) is regarded as the most important parameter in the biometric measurement of fibres (Curto, 2016).
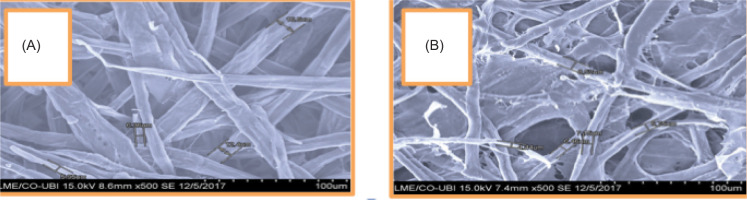
3.1.2. Morphological characterisation of the fibers using SEM
The results in Table 3 show two structures: the unrefined (a) and the refined (b), visualised using the SEM.
It can be seen that the unrefined structure (a) has retained its morphology. Following the refining treatment (b), fibrils can be visualised at the micro scale. This leads to the conclusion that the structure is microfibrillated and that refining has altered the morphology of the fibril walls, reducing their width.
3.1.3. Characterisation of leaf structures using ATR Fourier Transform Infrared Spectroscopy
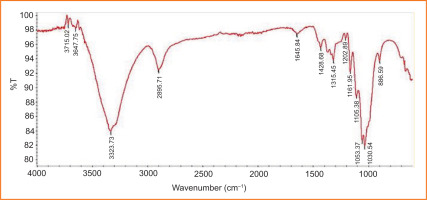
Using the FT-IR technique, it was possible to chemically identify the functional groups in the infrared spectra of the structures forming the matrix for the production of the spheres, using the spectrophotometer, as shown in Figure 9.
Note: FTIR-ATR enabled the determination of the presence or absence of these functional groups in the samples. However, since the raw material in both samples is the same (cellulose fibres) and the refining process does not alter the raw material chemically, we believe that chemical determination from a single image (figure) is sufficient and valid for both samples.
After analysing the unrefined and refined cellulose fibres (with 0.1% CMC additive), as shown in Figure 9, it can be concluded that functional groups are present in the sheets. The spectrum displays a broad peak between 3000 and 3500 cm–¹, typically indicating the presence of an OH functional group linked by hydrogen bonding. The region between 1200 and 1300 cm–¹ shows a band characteristic of alkyl ethers (C–O), while the 1400–1500 cm–¹ region corresponds to a band characteristic of double bonds. The band at around 1600 cm–¹ corresponds to the carbonyl group of an ester. Additionally, a band characteristic of tetrahedral carbon can be observed in the 2500–3000 cm–¹ region.
3.1.4. Porosity
The porosity of refined MFC (with added CMC) and unrefined MFC was determined from thickness and mass measurements, using cellulose density and known radius values, following the methodology described by Matko (2003) & Sereno et al. (2007):
1. Void volume=Total volume – Cellulose volume
2.
3. Total volume = Base area × Height
■ Vtotal = π × r2 × h being,
■ Vtotal = 3.14 × (8.1)2 × h being, h = thichness = 0.0194 cm
■ Vtotal = 3.14 × 65.610.0194 cm3
■ Vtotal = 3.996 cm3
Since porosity is determined from the thickness and mass values (Dullien, 1992 apud Sereno et al., 2007), knowing the density and radius values, the other parameters can be omitted.
Based on these results, and with the help of the Microsoft Excel programme, all the porosity values were calculated, as shown in Table 2.
Table 2
Results of the porosity of refined and unrefined MFC.
Table 2 shows that the unrefined pulps have higher porosity values than the refined sheets. This leads to the conclusion that the refining treatment reduces porosity. Therefore, pore dimensions are important in the drug delivery system because the smaller the pores, the greater the delay in drug release and, consequently, the more effective the clinical utilisation of the drug.
3.2. Kinetic studies of DDS
The kinetic studies enabled an understanding of the behaviour of the therapeutic molecules within the DDS and their interaction with the produced cellulose structures. These studies were conducted on DDS containing microfibrillated cellulose with incorporated therapeutic molecules—commercial Diclofenac, commercial Etoricoxib, and pure Diclofenac.
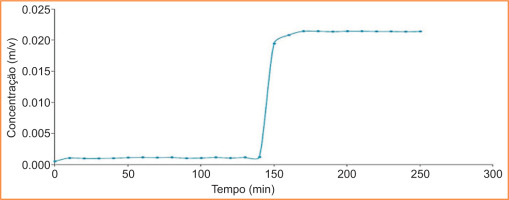
3.2.1. Kinetic studies of diclofenac from DDS with MFC
Figure 10 shows the kinetic study of the release of commercial Diclofenac from DDS with MFC. It can be seen that there was no release of the drug at pH 2 in the stomach (up to 120 minutes). From minute 130 onwards, a more abrupt release began, with the drug being released completely and at a certain rate. The abrupt release can be explained by the size of the pores in the structure formed with refined cellulose (MFC), as it is more open, given that the fibres are larger, with dimensions in the micrometre range (length of 800 μm and width of 19 μm). Similarly, complete disintegration can be explained by the presence of other constituents, which, although inert, can reduce the bonding between the fibres. After reaching the maximum peak, the concentration appears to decrease. However, according to the principle of conservation of mass, it remains constant in the test cup, and the oscillations can be explained by experimental errors associated with the replacement of the liquid when volume samples are taken for analysis by spectroscopy.
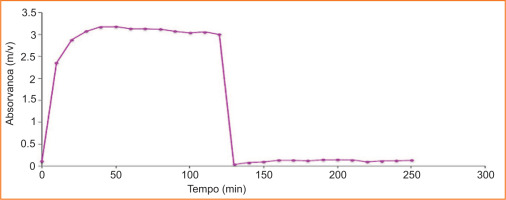
3.2.2. Etoricoxib kinetic studies of DDS with MFC
Figure 11 shows that the release profile of the Etoricoxib molecule is completely different from that of Diclofenac. In this case, drug release occurs at the stomach pH. Therefore, Etoricoxib retention is not effective.
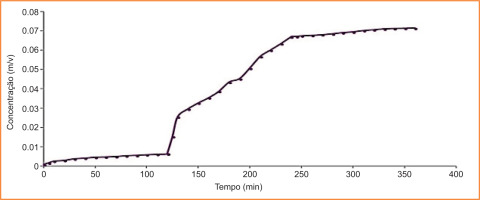
3.2.3. Kinetic studies of the release of analytically pure Diclofenac from DDS with MFC
Figure 12 shows the kinetic study of the release of analytically pure Diclofenac from DDS with MFC. It can be observed that the release profile of analytically pure Diclofenac with MFC closely resembles that shown in Figure 7, with minimal release at the stomach pH (pH 2, up to 120 minutes) and controlled release at the duodenal pH. However, commercial drugs contain other constituents (as detailed in their leaflets) which may influence the analysis. In this case, these additional components were considered inert.
3.3. Comparative studies between the NFC and MFC matrices in DDS
The MFC and NFC matrices exhibited similar controlled release behaviour at stomach pH; in both cases, there was no drug release at this acidic pH, aligning with the findings for NFC reported by Morais (2017). Some observed differences relate to porosity—specifically, MFC structures are more open, as the fibres have dimensions on the micrometre scale (800 μm in length and 19 μm in width). A key advantage of MFC is its ability to enable complete drug release at the pH of the small intestine (duodenum), indicating a degree of efficiency in delivering the drug into the bloodstream. However, while this rapid and complete release supports the effectiveness of the DDS, it also highlights a drawback: the release is not fully controlled, meaning the clinical effect is faster but potentially less sustained.
Note: When Once the drug is fully released, the alginate-based delivery system—being biocompatible and derived from algae—does not pose any risk to the body.
NFC matrices have fibre widths of approximately 40 nm. The resulting pores are smaller, and the hydrogen bonding between the OH groups of the nanofibrils creates a more compact matrix, thereby reducing pore size. As a result, drug release at the pH of the small intestine is more gradual. This slower, controlled release enhances the drug’s clinical utility within the body. However, limitations include the high production cost, due to the challenges in obtaining nanofibrils, as well as the technical difficulties associated with analysing structures at the nanoscale.
3.4. Modelling and chemical analysis of structures and therapeutic molecules
The 3D representations enabled a clearer visualisation of the spatial arrangement of atoms within each molecule, highlighting their conformational flexibility. They also allowed for observation of the volumes occupied by the electronic clouds of the respective molecules, as well as the various exposed functional groups that facilitate intra- and intermolecular bonding. In the case of the therapeutic molecules, the 3D models provided enhanced insight into their molecular structures, with Etoricoxib appearing more complex than Diclofenac.
3.5. Computer simulation study of structures and proposal of new DDSs
Simulation studies were carried out using MFC and NFC, demonstrating that the porosity of the structures can be modulated by varying the type of cellulose used. Future work could focus on producing hybrid structures combining MFC and NFC, followed by detailed characterisation. The results support the conclusion that similar outcomes can be achieved through computer simulation as with experimental structures. In other words, the simulated models closely replicate the laboratory-produced structures, exhibiting comparable porosity and pore distribution, as illustrated in Figure 13.
Figure 14
Example of a porous cellulose material, resulting from the simulator validated by Curto et al. (2011).

4. CONCLUSION AND PERSPECTIVES
The structures produced from refined pastes (MFC) are hydrophilic and exhibit different porosities compared to those obtained without refining or with NFC. Refining is essential for producing porous structures with more regular pore sizes, and both porosity and pore size play important roles in the kinetics of controlled drug release. Consequently, through the refining process, the MFC structures showed lower porosity than the unrefined ones, enabling the drug to be retained within this matrix. It was possible to produce DDS using microfibrillated cellulosic fibres that prevented the release of commercial Diclofenac in the stomach and enabled its release at intestinal pH. The controlled and complete release profile of the drug, without discharge at stomach pH, indicated the effectiveness of the developed systems in directing the drug into the bloodstream, thereby enhancing its clinical utilisation and highlighting its innovative functionality. The cellulose materials significantly influenced the release kinetics of Diclofenac, ensuring it not to be retained within the transport system. Kinetic studies conducted showed that the DDSs of the selected therapeutic molecules do not share an identical release profile, with Etoricoxib being released in the acidic pH of the stomach. Results from the computer simulation showed that similar outcomes can be achieved from experimental data using a computer simulator, thereby allowing structural optimisation with fewer experimental trials. The simulator offers various advantages, such as conserving time and experimental resources, and aiding in the understanding and prediction of fibre combinations for DDS production. Future work aims to design drug release systems (DDS) in which MFC and NFC are blended to achieve even lower porosity, thereby enhancing system efficiency. With a more efficient matrix, it will also be possible to incorporate essential oils for various medicinal purposes. Similarly, future studies are intended include in vivo testing on mice to better understand the biological interactions between DDS and drugs, thereby providing a clearer picture of potential human responses.
ACKNOWLEDGMENTS
This work was supported by the FibEnTech Research Unit, Department of Chemistry, Faculty of Sciences, University of Beira Interior, Portugal; Thank you very much! Our gratitude goes to Teacher Joana Maria Rodrigues Curto (PhD), our tutor, for all her support. Thank you very much!
AUTHORS CONTRIBUTION
Research concept and design: J.P.M.B.; Collection and/or assembly of data: J.P.M.B.; Data analysis and interpretation: J.P.M.B., N.M.H.; Writing the article: J.P.M.B., N.M.H., M.M.; Critical revision of the article: J.P.M.B., N.M.H., M.M., K-T-N.N.; Final approval of the article: J.P.M.B., N.M.H., M.M., K-T-N.N..