INTRODUCTION
Nephrolithiasis, commonly referred to as kidney stone disease, represents a globally prevalent affliction characterized by the formation of mineral concretions within the renal system. This malady exhibits varying incidence rates across diverse geographic regions, manifesting as approximately 1–5% in Asia, 7–13% in North America, and 5–9% in Europe (Sorokin, Mamoulakis, & Miyazawa, 2017). This rate is 12% in men and 5% in women (Hoopes, Reid, & Sen, 2003). The propensity for kidney stone formation is notably heightened during the summer season or in regions characterized by elevated temperatures. This phenomenon can be attributed to a reduction in overall bodily water content, culminating in the heightened concentration of urinary minerals. Consequently, this augmented concentration facilitates the sedimentation of diminutive mineral particles from the urine, ultimately culminating in the genesis of kidney stones (Sharma, Amin, & Prajapati, 2015). Calcium oxalate (CaOx) stones account for approximately 80% of kidney stones, while magnesium (Mg], ammonium phosphate, uric acid, hydroxyapatite, and cystine stones make up the remaining 20% (Bihl & Eyers, 2001).
Among the paramount challenges encountered in managing this ailment is the high recurrence rate of stone formation in patients, despite successful treatment of the detected stones, requiring new operations in the same patient (Koursh, Gordon, & Frank, 2004). Animal models serve as essential tools for comprehending the intricate mechanisms underlying the genesis of kidney stone formation and for establishing the empirical foundation of treatment modalities. The rat emerges as the preeminent and widely employed animal model in pursuit of these objectives (Bruijn et al., 1994; Esen, Akıncı, Aytekin, & Altuğ, 1990; Khan, 1997). Spontaneous kidney stone formation is sporadic in rats, and many different methods have been developed for experimental stone disease formation. These methods include oral ethylene glycol ingestion (EG], ascorbic acid intake, D3 vitamin, and intraperitoneal injection of 4-L hydroxyproline (Esen et al., 1990). CaOx stones, one of the most common kidney stones, can be formed quickly using EG and ammonium chloride (Khan, 1997). The anti-urolithiasis activity exhibited by this botanical specimen can be ascribed to a confluence of physiological mechanisms, including diuretic, antispasmodic, and antioxidant properties, alongside its capacity to exert inhibition on critical processes such as crystallization, nucleation, and crystal aggregation. These multifaceted mechanisms collectively contribute to ameliorating events and symptoms conducive to the genesis and advancement of renal calculi (Oswal et al., 2023). Urinary saturation is The primary event in stone formation. Saturation is followed by crystallization and nucleation. Factors affecting this chain are heat, pH, the presence of inhibitor complexes in the environment, and promoter substances. Some organic substances in the urine, such as citrate, combine with calcium to form calcium citrate. 'Citrate deficiency' in urine leads to the combination of existing calcium with oxalate, that is, the formation of CaOx stones (Arıkan, 2008) Studies have reported that the accumulation of CaOx in the kidney increases urinary pH, and this indication is a symptom of kidney stones (Takawale, Mali, Kapase, & Bodhankar, 2012). Viburnum opulus (VO) is a botanical specimen affiliated with the Viburnum L. genus within the taxonomic domain of the Adoxaceae botanical family. It is known as a snowball, cranberry, European cranberry bush, red elderberry, rose elder, cherry tree, cramp bark, guelder rose, and gilaburu in Turkey (Ilhan et al., 2014). The European cranberry (Viburnum opulus, VO) exhibits a broad distribution encompassing natural habitats spanning the expanse of the European continent. Moreover, its presence extends to select areas within North Asia and Northern Africa, along with a notable occurrence in the central reaches of Russia (Altun, Çitoǧlu, Yilmaz, & Özbek, 2009; Konarska & Domaciuk, 2018; Rop et al., 2010). The systematic location of the Viburnum opulus plant we used in our study is as follows; Branch Magnoliophyta, Class Magnoliopsida, Team Dipsacales, Family Caprifpliaceac, genus Viburnum, Species Viburnum opulus (Gilaburu) (Yıldız & Ekici, 2019). VO finds prevalent application in the realm of medicinal practices. Traditionally, VO has proactively mitigated certain gastrointestinal and renal disorders. Additionally, it has been harnessed for therapeutic interventions encompassing ailments like cough, cold, elevated blood pressure, diabetes, ulcerations, hepatic afflictions, rheumatic discomfort, and tuberculosis (Al et al., 2017; Altun et al., 2009; Eryılmaz et al., 2013; Kızılay et al., 2019; Sabur & Güneş, 2023; Česonienė, Daubaras, Kraujalytė, Venskutonis, & Šarkinas, 2014). It has been reported that the phenolic acid, ascorbic acid, and organic acid contained in the fruit of VO can reduce urinary pH and prevent the formation of kidney stones and that VO phenolics can modulate cellular membrane dynamics (Kajszczak, Zakłos-Szyda, & Podsędek, 2020; Małgorzata et al., 2019). An empirical investigation revealed that the oral administration of Vitis vinifera (VO) fruit extract, at concentrations of 10 mg/mL, in quantities of 0.5 and 1 mL, administered to rats over a duration of five days per week for a span of one month, elicited advantageous outcomes about the urinary system. The administration of said VO fruit extract engendered notable enhancements in urine citrate levels and volume, concurrently effecting reductions in urinary oxalate levels and urine cystine content. Moreover, discernible attenuation of crystal accumulation within renal tissue was observed. Notably, the extract exhibited a protective role against oxidative damage and the incipient formation of crystals within kidney tissue. The empirical findings in this context underscore the plausible efficacy of utilising the VO fruit extract as a prospective tool in mitigating mild to moderate hypocitraturia stone disease. These observations proffer a promising avenue for its consideration as a therapeutic intervention within the ambit of such clinical scenarios (Tuglu et al., 2014).
The primary objective of this investigation is to assess the prophylactic efficacy of Viburnum opulus juice in mitigating the development of experimentally induced kidney stone formations within a rat model.
MATERIALS AND METHODS
Collection and Extraction of Viburnum opulus
The VO fruits were obtained by traditional methods (Sağdıç, Yapar, Hayta, Sarıoğlu, & Yetim, 2013). The leaves and garbage in the collected gilaburu fruit were removed and washed with tap water. It was kept in a water container for two months, and the fruits were ripened. Then the fruits were weighed, and the fruits were pressed. Gillaburu juice was obtained and was concentrated under vacuum at 37-38 ºC using a rotavapor [Ilmvac vacuum pump, HeidolphLaborat 4000 efficient]. Approximately 58 g of dried fruit extract powder was obtained from 625 ml of fruit juice. All extracts were lyophilised (LabconcoFreezone 4.5) and stored at -20 ºC until use.
Determination of Dose Amount of Viburnum opulus Extract
Previous studies determined that the consumption of VO juice for therapeutic purposes in humans is approximately 250 ml, corresponding to about 1/8 of daily fluid intake (Ongan, 2018) . The daily water intake of rats was reported to be 10-12ml/100g (İde & Mülazımoğlu, 2012). Based on these calculations, a daily dose of approximately 1-1.5 g was calculated for the rats, dissolved in 2.5 ml of water, and administered to the rats by gavage.
Procurement of Experimental Animals
The research protocol underwent rigorous scrutiny and received ethical validation from the Erciyes University Animal Experiments Local Ethics Committee, as evidenced by the approval with reference number 2014/155. The study encompassed a cohort of 20 male Sprague Dawley rats, characterized by an average body weight of 250 grams each, thus forming the experimental foundation for the investigation. Each rat was taken from a conventional cage before the start of the study and placed in a metabolic cell, with one rat in each cell. The animal subjects were securely accommodated within traditional laboratory settings, characterized by meticulously maintained environmental parameters encompassing a controlled temperature of 22±1°C, humidity levels sustained at 50%, and adherence to a standardized light regimen of 12 hours of light followed by 12 hours of darkness. Nutritional needs were met through uniform rat diets, while access to tap water was afforded ad libitum, ensuring unrestrained sustenance availability.
Negative Control Group (n=6)
Fed a normal diet and not subjected to the kidney stone formation procedure.
Positive Control Group (n=7)
The kidney stone model was induced by adding 0.75% EG (EG) and 1% NH4Cl (AC) to the drinking water for eight days (Thatikonda, Rao, & Babu, 2013). The kidney stone formation procedure was applied.
Experimental group (n=7)
Drinking water was supplemented with 0.75% EG and 1% NH4Cl for eight days to induce kidney stone formation, and in addition, VO extract was administered to the animals by gavage for eight days starting from day 1 according to their body weight, to test its preventive effect on kidney stone formation.
Biochemical Analysis of Urine
The adaptation period of the experimental animals to the environment (three days) was followed by the start of the study. During the investigation, 24-hour urine samples were collected every day. Ca, uric acid, Mg, creatinine, oxalate, and citrate values were determined from urine samples collected on days 0, 4, and 8.
Collection and Biochemical Analysis of Blood Samples
Upon the culmination of the experimental trial, the rats were subjected to anaesthesia through an intraperitoneal administration of ketamine (80mg/kg) in conjunction with xylazine (5mg/kg). Subsequently, blood specimens were extracted from the aortic bifurcation site utilising a syringe. Urea, creatinine, Ca, and Mg values were determined from the collected blood samples.
Statistical Analysis
The acquired data underwent comprehensive analysis employing the SPSS 22.0 statistical software (SPSS, Inc., Chicago, Illinois, USA) within a computational framework. Daily water intake, urine volume, Ca, creatinine, Mg, uric acid, oxalate, and citrate values in urine were recorded separately for each rat using the SPSS statistical analysis program for days 0, 4, and 8 for the experimental group. In addition, Mg, creatinine, uric acid, and Ca values in blood were statistically compared. Descriptive statistics were initially applied to all data of the study. Descriptive statistics were employed to present the central tendency and variability of the measured variables, with mean values and standard deviations being reported. Subsequently, a normality assessment was conducted using the Shapiro-Wilk test, revealing a departure from a normal distribution pattern (p<0.05) within the dataset. This determined that non-parametric statistical analyses were more appropriate for the ensuing investigation.
The Mann-Whitney U Test was selected for binary comparisons, while comparisons involving more than two groups were subjected to the Kruskal-Wallis Test. A threshold of statistical significance was set at a p-value of <0.05. Findings were reported as mean values accompanied by their corresponding standard deviations. Notably, a significance level of p<0.05 was deemed acceptable for all statistical tests conducted throughout the study.
RESULTS:
Rat weights, daily water consumption, and daily urination
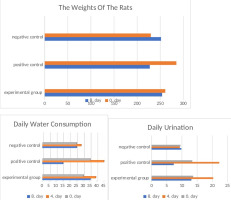
The anthropometric measurements of the subjects in the experimental clusters were assessed at two distinct time points: the study's inception (designated as day 0) and the conclusion of the specified period (marked as day 8). This comprehensive evaluation of weight variations provides insights into the subjects' physiological dynamics throughout the experiment. Weight gain was statistically significant in all negative control animals (p<0.05). A noteworthy reduction in body weight was observed within the positive control group. Conversely, the experimental group exhibited a marginal average weight loss that did not achieve statistical significance (p>0.05). This intriguing observation suggests a potentially protective influence conferred by VO water, as evidenced by the absence of discernible weight fluctuations within this cohort. Such findings warrant further exploration into the putative impact of VO water on weight management and metabolic dynamics (Figure 1).
Biochemical values of urine
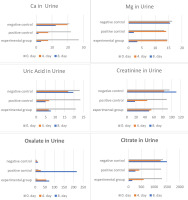
Biochemical analysis findings in urine were determined, as shown in Figure 2. A noteworthy surge in calcium and creatinine levels was evident when juxtaposed with the positive control, demonstrating statistical significance. Concurrently, a substantial reduction in oxalate levels was statistically discernible. Regarding citrate values, a comparative analysis between the positive control and the experimental groups revealed a statistically significant lower mean value within the experimental cohort, signifying a directional decrease (p<0.05). This multifaceted analysis sheds light on the distinct biochemical alterations induced by the empirical conditions of the positive control (Figure 2).
Biochemical values of serum
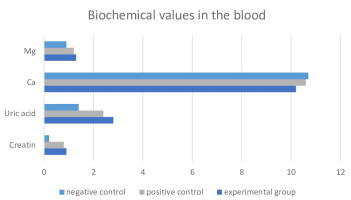
On the 8th day of the experiment, biochemical analyses were performed on blood samples obtained from animals in each group, and the values of Creatinine, Uric Acid, Ca, and Mg were measured. The mean values of the findings are given in Figure 3. In contrast to the negative control group, both the positive control and experimental groups exhibited a statistically noteworthy elevation (p<0.01) in levels of Creatinine, Uric Acid, Calcium (Ca), and Magnesium (Mg). Nonetheless, an absence of statistically significant differentiation emerged between the positive control and experimental groups.
Histopathological Findings
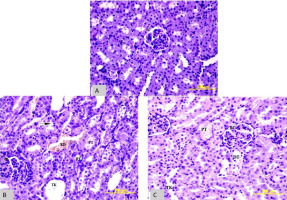
Histological sections were meticulously extracted from the tissue samples of all three cohorts of animals. These sections underwent staining procedures utilising hematoxylin-eosin (HE) to elucidate histopathological alterations and anatomical structures within the tissue. Furthermore, the Pizzolato (PZ) staining technique was employed to capture the manifestation of stone formation visually, thus facilitating a comprehensive histological assessment of the tissue specimens. When the kidney sections stained with HE from the positive control group were compared with the negative control group, structural damage in the proximal tubules and dispersed cells was observed in the renal cortex. Additionally, enlargement in the collecting ducts and necrotic cells was observed. Although vacuolisation and cellular damage were observed in the experimental group, both proximal and distal tubules were similar to the negative control. When compared to the positive control group, the damage was less. Vacuolisation was present in both proximal and distal tubule cells, but the number of necrotic cells was lower (Figure 4).
Figure 4
Comparison of HE stainingand tissues in A- Negative control, B-positive control C-experimental group.BC- bowman capsule, DT- distal tubule, TK- collecting duct, PT- proximal tubule, KD- blood vessel. Magnification*100
After staining kidney sections of both the positive control and experimental groups of animals using the PZ method (Figure 5), four different regions from one kidney section of each animal were counted by three other researchers at a 10X magnification, and the average was taken (Table 1). The average number of stones in one area of the positive control group was 17.64, while the average number in the experimental group was 7.27 (Figure 6).
Figure 5
Comparison of tissueswith PZ staining in A negative control, B positive control and C experimental group Magnification*500
Figure 6
Distribution of meannumber of stones in the positive control group and the experimental group
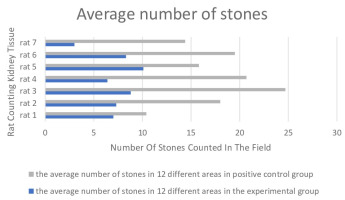
Table 1
Table display of the number of stones counted in random areas for each researcher
DISCUSSION
The incidence of urinary stone disease displays geographic and ethnic variations on a global scale, and its prevalence demonstrates a concomitant rise that correlates with the progression of socio-economic development. A substantial body of evidence highlights the predominant role of calcium oxalate (CaOx) as the primary constituent in forming kidney stones, wherein hyperoxaluria emerges as a notable, influential element in instigating the onset of CaOx kidney stone crystallisation. This correlation underscores the intricate interplay involving geographical distribution, genetic predisposition, and individual lifestyle choices, collectively contributing to the complex patterns observed in the occurrence and composition of urinary stone disease (Kulaksızoğlu & Gözlükaya, 2003). Urinary system stones recur at an average rate of 50% over ten years if no action is taken (Koursh et al., 2004). The mechanism of kidney stone formation is not fully understood, and many theories have been put forward, and experimental studies have been conducted to prove these theories. The importance of animal models in elucidating every process that plays a role in the pathogenesis of stone formation in the kidneys is significant, and one of the most studied animals is the rat. Since the most common kidney stone in humans is CaOx, the CaOx model is frequently used in studies. CaOx stones are acutely or chronically formed in rats by inducing hyperoxaluria (Khan, 1997).
In the literature, kidney stones have been experimentally formed in rats using EG and AC at different concentrations (Atmani & Silimani, 2003; Bashir, Gilani, & Siddiqui, 2010; Fan, Glass, & Chandhoke, 1999; Khan, 1997; Thatikonda et al., 2013; Touhami, Laroubi, & Elhabazi, 2007; Yamaguchi et al., 2005). In our study to investigate the protective effect of a herbal fruit juice, kidney stones were formed by adding 0.75% EG and 1% AC to drinking water for eight days.
Various studies (Yılmaz, Ekinci, & Ömerli, 2023) have been conducted in the literature to treat or prevent kidney stone disease through herbal ways. It has been reported that herbal products have no side effects (Thatikonda et al., 2013). In recent years, some researchers in our country have studied the impact of VO juice on kidney stones and reported positive results on experimentally formed kidney stones (Erdem, Kesik, & Honca, 2016; Ilhan et al., 2014; Çapkın & Fakültesi Üroloji Anabilim Dalı, 2014). They found a statistically significant difference in urinary oxalate and citrate levels with VO supplementation. They also showed that it could prevent hyperoxaluria and positively affect existing kidney stones. In terms of the mechanisms that could be effective, the importance of increasing urine citrate levels and decreasing CaOx levels has been emphasised (Çapkın & Fakültesi Üroloji Anabilim Dalı, 2014). In another study, the group given VO juice had increased urine volume, reduced cystine and oxalate levels compared to other groups, and increased citrate levels. In the positive control group, urine levels of Ca, uric acid, and phosphate increased, while they decreased in the VO group. A discernible disparity emerged in kidney weight, with the positive control group exhibiting elevated kidney weight compared to the remaining groups. Conversely, the gilâburu-treated group demonstrated a diminished kidney weight relative to the positive control, albeit still surpassing the kidney weight observed in the negative control group. When the sections stained with HE were evaluated histologically, vacuolar changes in the epithelial cells of the tubules, flattening of the epithelial cells, transparency in some areas, and intense crystal accumulation were found in the positive control group. In the VO group, similar changes were observed in the epithelial cells of the tubules, but they were less pronounced and closer to normal. They also reported that crystal accumulation was less in this group than in the positive control group (Erdem et al., 2016).
In our study, the experimental group had higher water consumption and urine volume than the positive and negative control groups. We can attribute this to the diuretic effect of the VO given to this group, as the urine volume was higher than the others. The average weight change in the experimental group was similar to that of the negative control group, suggesting that VO water prevented weight loss in animals. Since the weight changes in the experimental and hostile control groups were similar, VO juice reduces the effect of EG+AC and prevents weight loss. In the experimental group, the values of Ca, Mg, uric acid, and creatinine were higher than those of the positive control group and similar to those of the negative control group. The increase in Ca excretion in the experimental group indicates that the Ca ions were not bound and were excreted, leading to a decrease in crystal accumulation. The reduction in Mg values was related to increased CaOx stone formation and CaOx crystallisation. Urine creatinine testing is an essential test for evaluating and diagnosing kidney function. An increase in creatinine values is considered a sign of infection, while a decrease in creatinine values indicates a disturbance in kidney function. The higher creatinine values in the experimental group than in the positive control group suggest that VO water has a therapeutic effect on kidney structure. Based on these results, we can conclude that VO water has a therapeutic impact on biochemical values. An increase in oxalate levels in urine is one of the factors that increase the risk of urinary system stone formation. The changes in oxalate values in our experiment are crucial in interpreting the protective effect of VO water. The increase in the positive control group is more than three times that of the experimental group. Therefore, it can be said that VO water reduces the risk of urinary system stone formation as the oxalate excretion in the group given VO water was approximately three times less than that of the positive control group. The citrate value in the experimental group was lower than that of the positive control group, indicating that VO water did not have a citrate-increasing effect in the experimental group. Citrate is an inhibitor of urinary calcium stones. Its low excretion increases the risk of kidney stone formation. Citrate binds with Ca and inhibits the growth of crystals. Based on these results, it can be concluded that VO water has an increasing effect on citrate and that an increase in citrate levels can prevent crystal formation as citrate ions inhibit the formation of CaOx crystals (Tanagho & Mcaninch, 2000). In the experimental group, the blood's average values of Mg, Ca, uric acid, and creatinine were higher than those of the positive and negative control groups. VO water has a positive effect on increasing these values. When histological sections were examined and evaluated regarding kidney structural conditions using the HE staining method, a more normal appearance was observed in the experimental group compared to the positive control group. It can also be said that VO water can prevent the deterioration of histological structure. When sections stained with the PZ method were counted for stones in the experimental group, it was determined that the number of rocks in the positive control group was 2.5 times higher than in the experimental group. Therefore, VO water prevents stone formation.
Conclusion
The mechanism of action of herbal treatments is slower, so more definitive results can be obtained by giving VO juice for at least 30 days. In the method we used to create kidney stones, EG makes kidney stones over a long period, while AC is a component that accelerates stone formation. When used together, it can cause kidney failure and death by damaging the renal parenchyma after the 9th day. More definitive results can be obtained by conducting this study by providing only EG and Gavaj with VO water for 30 days.