1. INTRODUCTION
For millennia, traditional medicine has relied heavily on medicinal plants, in which the discovery of novel therapeutic agents hold enormous promise. However, the great biodiversity and the intricacy of plant chemistry provide formidable scientific hurdles. The emergence of artificial intelligence (AI) technologies provides creative answers to these problems, allowing scientists to predict biological activity, evaluate enormous datasets, and expedite the drug discovery process.
There are several advantages and breakthroughs when AI is used in medical plant research, especially when it comes to drug development and discovery. The methods used to detect and analyze the bioactive chemicals found in medicinal plants have changed as a result of AI, particularly deep learning (DL). In contrast to the more conventional machine learning (ML) techniques like support vector machines (SVMs) and random forests (RFs), the HerbMet platform, for example, uses DL to improve metabolomics data analysis, allowing for more accurate identification of Chinese herbal remedies (Sha et al., 2025). The advancement of herbal medicine depends on this ability to automatically extract unique properties from complicated datasets.
Furthermore, AI plays a part in all phases of drug development, not just identification, and its applications speed up the entire drug development process by facilitating molecular design, drug screening, and clinical trial process optimization (Harrer et al., 2019; Kolluri et al., 2022). In the case of traditional medicinal plants, which frequently contain multiple active compounds, AI’s capacity to analyze large datasets makes it possible to identify novel drug candidates and predict their interactions (Sharma et al., 2013; Li et al., 2011). For instance, by combining several data sources to forecast the safety and effectiveness of possible drug candidates, AI-driven platforms such as MolProphet expedite the early phases of drug discovery (Yang et al., 2024).
AI in medicinal plant research has several advantages beyond efficiency, such as increased accuracy and lower expenses. Given the high attrition rates in pharmaceutical research, researchers can reduce the time and resources typically needed for medication development by utilizing AI algorithms (Vora et al., 2023). Additionally, AI can increase the accuracy of medication formulations made from medicinal plants, guaranteeing that side effects are reduced and therapeutic results are increased (Amabie et al., 2024; Wang et al., 2023). This is especially pertinent to personalized medicine, where AI can assist in customizing therapies according to the unique characteristics of each patient, enhancing therapeutic results (Zeb et al., 2024).
There are still issues with AI in medicinal plant research, especially with regard to data quality and ethical issues, despite the technology’s encouraging uses. The availability of various high-quality datasets that appropriately reflect the complexity of medicinal plant chemicals and their interactions is essential for the successful application of AI technology (Kapustina et al., 2024). To guarantee fair access to these developments in healthcare, ethical issues pertaining to data privacy and the possibility of bias in AI algorithms must also be resolved (Ueda et al., 2024).
To sum up, the use of AI in medicinal plant research has revolutionary potential for the production of new drugs and the potential to completely transform the area by improving detection and analysis of bioactive substances, expedite the drug development process, and increase the precision and economy of therapies. For these technologies to be successfully incorporated into standard medical procedures, it is imperative to address the issues of data quality and ethical considerations.
For millennia, people have utilized medicinal plants to treat a variety of illnesses and enhance general health. Nevertheless, conventional research techniques for determining and evaluating therapeutic qualities are sometimes labor-intensive and time-consuming. Research on medicinal plants is undergoing a revolution thanks to the incorporation of AI, which is speeding up discovery and improving the extraction and use of plant-based chemicals. This article outlines the major advantages of this technological breakthrough and examines important AI applications in medicinal plant research.
2. MATERIALS AND METHODS
The scientific databases searched were PubMed, Scopus, Web of Science, ScienceDirect, Google Scholar, Springer, and Wiley using MeSH (Medical Subject Headings) terms such as “medicinal plants,” “artificial intelligence,” “plant identification and classification,” “ecological monitoring,” “bioactive substances,” “phytochemical analysis,” “drug discovery and virtual screening,” “data mining,” “personalized medicine,” “disease treatment and drug discovery,” “ethnopharmacology,” “machine learning,” “characteristics/anatomical, morphological, phytochemical, and molecular genetics,” “deep learning,” “natural language processing,” “ethnobotany,” “disease prediction with AI,” “treatment optimization with AI,” “field survey methods,” “predictive modeling,” and “clinical trials.”
Inclusion requirements include:
Studies that explore both modern research and traditional uses of medicinal plants, with a focus on utilizing AI, ML, and data mining techniques.
Papers proposing methods to determine the medicinal plants using AI-driven tools for classification, identification, phytochemical analysis, drug discovery, and virtual screening.
Including their potential mechanisms of influence on plant species, with a focus on AI-based modeling, predictive modeling, and data analytics for studying medicinal plants.
Studies on phytochemical analysis and bioactive substances in medicinal plants, particularly those incorporating AI-assisted virtual screening and drug discovery to identify novel compounds.
Papers applying AI techniques for drug discovery, virtual screening, or phytochemical analysis to identify promising plant-based compounds for therapeutic use.
Research on ethnopharmacology and the use of AI for analyzing traditional knowledge, especially with the application of NLP to extract relevant information from ethnobotanical sources.
Studies exploring the use of AI for disease prediction, treatment optimization, and personalized medicine based on medicinal plant properties.
Studies published in English, Kazakh, or Russian languages that utilize AI techniques in the context of medicinal plant research.
Exclusion criteria include:
Duplicate and incomplete information
Abstracts
Letters to the editor
Experiments employing linked homeopathic medications
The plant’s chemical components were discovered, and Chem Spider and PubChem were used to confirm their IUPAC (International Union of Pure and Applied Chemistry.) designations, structural formulas, and chemical formulas. According to WFO (World Flora Online), the plant’s taxonomy has been verified.
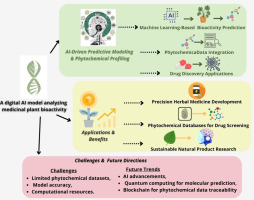
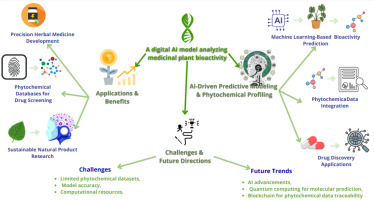
By analyzing important AI-driven approaches and their uses in the identification, categorization, and therapeutic use of plant-based substances, this study was aimed toward investigating the revolutionary role of AI in furthering medicinal plant research. Time-consuming procedures that frequently depend on manual plant identification, arduous phytochemical analysis, and prolonged clinical validation have constrained traditional approaches to medicinal plant research. A groundbreaking method for overcoming these obstacles is the incorporation of AI technologies like ML), DL, and NLP, which greatly improves the speed, accuracy, and scalability of research on medicinal plants.
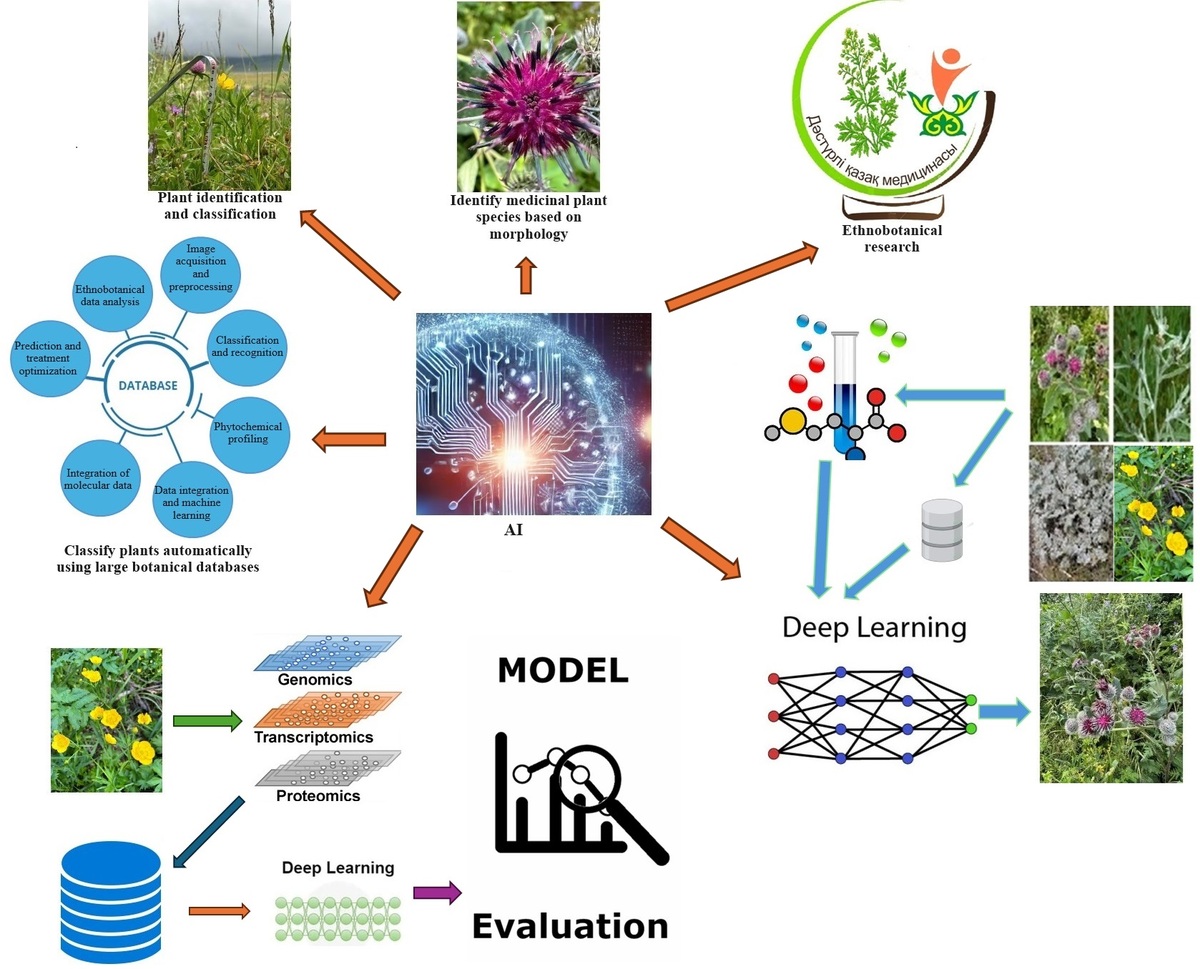
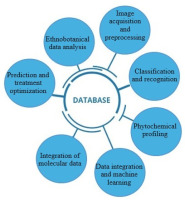
This work explores AI-based methods for identifying bioactive chemicals from medicinal plants, predicting bioactivity, and classifying plants. In order to help create a more effective drug discovery and virtual screening procedures, the focus is on how AI tools can simplify the identification of plant species based on morphological traits, chemical markers, and molecular genetics. In keeping with the rising demand for precision healthcare, this study also emphasizes AI’s ability to optimize disease prediction models, treatment plans, and tailored medicine utilizing medicinal plants (Figure 1).
This study also highlights how AI and traditional ethnobotanical knowledge overlap, showing how AI may help preserve and incorporate this priceless cultural legacy into contemporary medical research, and offers insights into how AI may improve biodiversity evaluations, increase agricultural output, and aid in the protection of endangered plant species by combining data from important scientific databases. In conclusion, the goal of this work was to highlight the transformative potential of AI in medicinal plant research, which will enable quicker and more precise plant species identification and classification while also propelling advancements in personalized medicine, drug development, and treatment optimization. The ultimate goal was to open the door for fresh, AI-powered methods that should increase healthcare results while simultaneously supporting long-term ecological preservation and traditional knowledge preservation.
3. PLANT IDENTIFICATION AND CLASSIFICATION
AI can assist in correctly identifying medicinal plants from photos, especially through ML and computer vision. AI algorithms can identify uncommon or endangered plants and categorize species by examining trends in plant characteristics, such as leaf shape, color, and flower structure. This can greatly expedite database construction and fieldwork.
The use of AI in medicinal plant identification and categorization has become a game-changer, improving the precision and effectiveness of conventional techniques. Automating the classification of different herbal remedies has shown great promise thanks to AI approaches, especially DL. In a profession where precise identification is essential in guaranteeing the effectiveness and safety of herbal therapies, this development is critical. To overcome the difficulties posed by high-dimensional data in the categorization of herbal medicines, new techniques, including Mutual Triplet Attention Learning (MTAL), have been developed in addition to DL. This approach shows how AI approaches can be tailored to the unique requirements of herbal medicine research by combining locally linear embedding (LLE) and linear discriminant analysis (LDA) to increase classification accuracy (Liu et al., 2013). Additionally, to demonstrate the adaptability of AI in managing a variety of data types, AI-driven image processing algorithms have been used to identify and categorize the leaves of several medicinal plant species (Hajam et al., 2024). These developments lessen the need for expert knowledge, which can be arbitrary and inconsistent, while also streamlining the classification process. Furthermore, the development of standardized herbal medicine practices will be significantly impacted by the incorporation of AI into the classification of medicinal plants. Researchers can create more objective categorization criteria by using AI algorithms, which is crucial for quality control and regulatory compliance in the manufacturing of herbal medicines. For instance, categorizing herbal medications according to their aromatic profiles using electronic nose devices in conjunction with AI algorithms has been investigated, offering a quick and accurate classification technique (Kavitha et al., 2023). With the growing need for herbal treatments worldwide, this method improves the capacity to assess and classify herbal goods. Beyond the classification accuracy, AI’s advantages in medicinal plant identification also include the possibility of extensive data integration and analysis. Large datasets from multiple sources, including genetic, environmental, and phenotypic data, can be analyzed by AI systems to find patterns and linkages that conventional approaches would miss. Understanding the unique properties of medicinal plants might result in more specialized therapeutic uses, making this expertise especially useful in the context of personalized medicine (Thomford et al., 2018). Notwithstanding the encouraging developments, there are still difficulties in using AI to the classification of medicinal plants. To get the most out of these technologies, problems with data quality, the requirement for large training datasets, and the interpretability of AI models must be resolved (Kumar et al., 2022). Additionally, to guarantee fair access to AI-driven solutions in herbal medicine research, ethical concerns about data privacy and the possibility of bias in AI algorithms demand constant examination (Ahmadi and Ganji, 2023). In summary, a major advancement in the subject has been made with the use of AI into the identification and classification of medicinal plants. AI technologies have the potential to completely transform the classification and use of herbal medicines by improving accuracy, efficiency, and uniformity. This could ultimately lead to better health outcomes and the development of personalized medicine.
A significant challenge in medicinal plant research is the accurate identification and classification of plant species. Misidentification can lead to ineffective or even harmful medicinal use.
AI-powered tools such as computer vision and convolutional neural networks (CNNs) can.
3.1. Analyze plant morphology through image recognition
Plant morphology, the study of the form and structure of plants, is essential for understanding plant diversity, evolution, and ecology. Traditional methods of morphological analysis often involve manual measurements and visual assessments, which can be time-consuming and subject to human error. The advent of AI, particularly through DL and image recognition technologies, offers a promising alternative that can automate and enhance these processes. Recent studies have demonstrated the efficacy of CNNs in identifying and classifying plant species based on morphological characteristics extracted from images.
3.1.1. Methodologies in AI-driven plant morphology analysis
The methodologies in AI-driven plant morphology analysis have evolved significantly, leveraging advancements in ML, computer vision, and DL techniques. These methodologies are crucial for enhancing our understanding of plant traits, optimizing agricultural practices, and addressing challenges in food security.
One of the primary methodologies involves the use of ML algorithms to analyze plant phenotypes. Gupta et al. (2024) highlight that AI technologies automate the measurement and analysis of plant characteristics, facilitating real-time monitoring of plant health and growth, which is essential for innovative research and improved crop management practices. This automation allows for the efficient collection of large datasets, which can be analyzed to uncover meaningful patterns in plant growth and adaptation mechanisms (Singh et al., 2016). Furthermore, the integration of DL techniques, such as CNNs, has shown significant promise in accurately identifying plant species and assessing their health (Ahmad et al., 2023; Mulugeta et al., 2024). These approaches not only enhance the precision of plant trait analysis but also contribute to the development of predictive models that can inform agricultural decision-making.
In the context of hydroponics and soilless farming, AI-driven methodologies have been employed to optimize growing conditions. The development of smart hydroponics systems utilizing AI-based sensing techniques exemplifies this trend. Putra et al. (2024) discuss how intelligent agents can interpret plant health data to adjust environmental conditions dynamically, thereby improving growth outcomes. Additionally, the AI-driven pheno-parenting model proposed by Hati and Singh (2023) employs DL for real-time analysis of plant traits, further enhancing the understanding of plant life cycles and growth optimization. Such methodologies not only improve yield but also contribute to sustainable agricultural practices by minimizing resource use and maximizing efficiency.
Moreover, explainable AI (XAI) is emerging as a vital component in plant phenotyping, providing interpretable insights from complex DL models. Mostafa et al. (2023) emphasizes the importance of XAI in making the results of DL models trustworthy and understandable for plant scientists, which is crucial for integrating these technologies into practical applications. This transparency is essential for fostering confidence in AI-driven methodologies among researchers and practitioners in the field. XAI methods, such as Grad-CAM or LIME, allow visualization of which specific image regions influenced the model solution, which ensures interpretability of the results. This is especially important in an interdisciplinary context where results need to be understandable to both botanists and ML experts.
3.1.1.1. Image acquisition and preprocessing. The first step in AI-driven plant morphology analysis involves acquiring high-quality images of plant specimens. This can be achieved through various imaging techniques, including digital photography and specialized imaging systems. Preprocessing steps, such as normalization, resizing, and augmentation, are crucial to preparing the images for analysis. These steps enhance the robustness of the AI models by ensuring that they can generalize well across different conditions and variations in plant morphology (Huixian, 2020; Taslim et al., 2021).
3.1.1.2. Feature extraction using deep learning. DL models, particularly CNNs, have shown remarkable success in feature extraction from images without the need for manual feature engineering. For instance, studies have utilized CNNs to automatically identify morphological traits such as leaf shape, size, and texture (Taslim et al., 2021; Fraiwan et al., 2022). The ability of CNNs to learn hierarchical features makes them particularly suited for complex image datasets where traditional methods may falter.
3.1.1.3. Classification and recognition. Once features are extracted, AI models classify plant images into different species or identify specific morphological traits. Research has demonstrated that AI systems can achieve high accuracy rates in plant classification tasks, often surpassing traditional methods (Huixian, 2020; “Classification of plant leaf diseases using deep neural networks in color and grayscale images,” 2024). For example, a study employing a CNN-based approach for leaf identification reported an accuracy rate of over 85% (Huixian, 2020). Furthermore, AI can assist in recognizing subtle morphological variations that may be indicative of specific genetic traits or environmental adaptations.
3.1.2. Applications and benefits of AI in plant morphology analysis
The application of AI in plant disease detection further illustrates the transformative potential of these methodologies (Figure 2). AI-driven image recognition techniques have been reported to achieve accuracies exceeding 95% in automated disease detection, significantly outperforming human assessments (González-Rodríguez, 2024; Sahoo, 2024). These methodologies enable early disease identification, which is critical for effective crop management and minimizing losses. The integration of weather, soil, and crop data into forecasting models enhances the ability to predict disease outbreaks, allowing for timely interventions (González-Rodríguez, 2024; Sahoo, 2024). This proactive approach not only improves crop resilience but also supports sustainable agricultural practices by reducing the reliance on chemical treatments.
3.1.2.1. Biodiversity assessment. NAI-driven image recognition facilitates large-scale biodiversity assessments by enabling researchers to analyze vast quantities of plant images efficiently. This capability is particularly beneficial in ecological studies where rapid identification of species is necessary for monitoring changes in biodiversity and ecosystem health (Younis et al., 2018).
3.1.2.2. Agricultural productivity. In agriculture, AI can be employed to monitor crop health and identify morphological traits associated with yield potential. By analyzing leaf images for signs of disease or nutrient deficiencies, AI systems can provide timely insights that help farmers make informed decisions, ultimately enhancing productivity (Sunandar, 2024). For instance, the classification of leaf diseases using AI has proven effective in improving crop management strategies (Classification of plant leaf diseases using deep neural networks in color and grayscale images, 2024).
3.1.2.3. Conservation efforts. AI technologies can also play a crucial role in conservation biology by aiding in the identification of endangered plant species and monitoring its population. By automating the analysis of herbarium specimens and field images, researchers can gather data on species distribution and health more efficiently, contributing to better conservation strategies (Younis et al., 2018).
3.1.2.4. Challenges and future directions. Despite the promising advancements, several challenges remain in the application of AI for plant morphology analysis. These include the need for large annotated datasets to train AI models effectively, the potential for bias in model predictions, and the requirement for interdisciplinary collaboration between botanists and data scientists (Huixian, 2020; Taslim et al., 2021). Future research should focus on developing more robust models that can operate with limited data and exploring the integration of AI with other technologies, such as remote sensing, to enhance plant morphology analysis further.
In summary, the methodologies in AI-driven plant morphology analysis encompass a range of advanced techniques that enhance our understanding of plant traits and optimize agricultural practices. The integration of ML, DL, and XAI into these methodologies provides researchers with powerful tools to analyze complex plant data, improve crop management, and address challenges in food security.
The integration of AI in analyzing plant morphology through image recognition represents a significant advancement in botanical research. By automating and enhancing morphological assessments, AI technologies can facilitate biodiversity studies, improve agricultural practices, and support conservation efforts. Continued research and development in this field will undoubtedly lead to more refined methodologies and broader applications, ultimately contributing to our understanding and management of plant diversity.
3.2. Identify medicinal plant species based on leaf patterns, flowers, and chemical markers
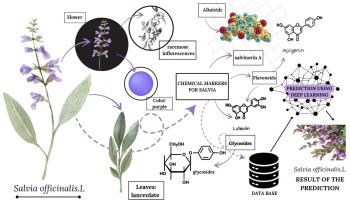
The identification of medicinal plant species based on leaf patterns, flowers, and chemical markers is a critical area of research that has gained traction with the advent of AI technologies. This interdisciplinary approach combines traditional botanical knowledge with modern computational techniques to enhance the accuracy and efficiency of plant species identification. One of the primary methodologies employed in this domain is the use of image processing techniques to analyze leaf morphology. Leaves are often the most accessible and visually distinctive parts of plants, making them a focal point for identification. Arun and Durairaj (2017) emphasize the importance of utilizing texture and color space analysis in leaf identification, which can be effectively integrated into AI systems for automatic recognition of medicinal plants. This method allows for the extraction of unique features from leaf images, which can then be classified using ML algorithms. The development of specialized databases, such as the MepcoTropicLeaf, enhances the training of these algorithms by providing annotated images of Indian medicinal plants (Priyadharshini et al., 2021). In addition to morphological analysis, chemical markers play a significant role in the identification of medicinal plants. Chemical profiling, including the analysis of secondary metabolites, provides insights into the unique biochemical signatures of different species. For instance, Rivera-Mondragón et al. (2017) discuss the use of high-performance liquid chromatography (HPLC) to quantify phenolic compounds in Cecropia species, highlighting the importance of chemical markers in quality control and species differentiation. Furthermore, the integration of DNA (deoxyribonucleic acid) barcoding with chemical analysis has been shown to enhance the reliability of species identification. Palhares et al. (2015) assert that combining DNA barcoding with chemical fingerprinting ensures the quality of herbal medicines by confirming species identity and the presence of active compounds. The use of molecular markers, particularly DNA barcoding, has revolutionized the identification process by providing a genetic basis for distinguishing between closely related species. Mishra et al. (2015) note that while morphological traits are valuable, they may not always suffice for accurate identification, especially in cases of adulteration or misidentification in the herbal market. DNA barcoding offers a robust solution by utilizing specific genetic sequences to authenticate plant species, thus addressing challenges in the herbal industry (Ganie et al., 2015). The chloroplast genome, as highlighted by Xia et al. (2022), serves as an effective DNA super barcode for the identification of medicinal plants due to its conserved nature and phylogenetic significance. AI technologies also facilitate the integration of various data types, including morphological, chemical, and genetic information, into comprehensive identification systems. The work by Thella and Ulagamuthalvi (2021) illustrates the potential of AI-driven classification models that utilize leaf images for accurate medicinal plant detection, underscoring the importance of automated systems in enhancing efficiency in plant identification. These models can be trained on diverse datasets, allowing for the recognition of patterns that may not be immediately apparent through traditional methods. In summary, the identification of medicinal plant species through AI-driven methodologies that incorporate leaf patterns, flowers, and chemical markers represents a significant advancement in botanical research. The combination of image processing, chemical profiling, and molecular techniques provides a multifaceted approach to plant identification, ensuring accuracy and reliability in the classification of medicinal plants (Figure 3).
Figure 3
Methodologies in AI-driven medicinal plant species based on leaf patterns, flowers, and chemical markers.
3.2.1. Methodologies for AI-driven identification
The methodologies for AI-driven identification of medicinal plant species based on leaf patterns, flowers, and chemical markers have gained significant traction in recent years. These methodologies leverage advanced computational techniques, including ML and DL, to enhance the accuracy and efficiency of plant identification processes. One of the foundational methodologies in this area is the use of image processing and ML algorithms for the identification of medicinal plants. Gokhale et al. (2020) emphasize the importance of image processing techniques combined with ML to accurately classify medicinal plants based on visual characteristics such as leaf shape, color, and texture. This approach not only facilitates the identification of plant species but also aids in distinguishing between closely related species that may have similar morphological traits. The integration of CNNs has further enhanced the classification accuracy, as demonstrated by Goyal (2023), who reported a remarkable 99% accuracy in distinguishing between multiple medicinal plant species using optimized DL models. Current research demonstrates that pretrained models such as ResNet, EfficientNet, and DenseNet have been successfully applied for automated identification of medicinal plants from leaf images. According to the study by Goyal (2023), in a comparative analysis of seven models, including VGG16, VGG19, DenseNet201, ResNet50V2, Xception, InceptionResNetV2, and InceptionV3, DenseNet201 demonstrated the highest accuracy (up to 99.64% for public data) by optimizing the ratio of parameter counts and computational efficiency. In particular, the ResNet architecture, due to its residual links, allows efficient learning even in deep networks, while EfficientNet provides high accuracy at lower computational cost, as confirmed by the results of this study. In addition to morphological analysis, the incorporation of chemical markers plays a crucial role in the identification of medicinal plants. Palhares et al. (2015) highlight the necessity of combining DNA barcoding with chemical analyses to ensure the quality and authenticity of medicinal plants. This dual approach allows for a more comprehensive understanding of plant identity, as chemical profiles can reveal the presence of specific bioactive compounds that are characteristic of certain species. Furthermore, Lu et al. (2022) discuss the pharmacophylogenetic relationships among medicinal plants, suggesting that morphological characteristics, chemical markers, and DNA sequences should be integrated to infer phylogenetic relationships accurately. This holistic approach not only aids in species identification but also provides insights into the evolutionary relationships among plants. The application of DNA barcoding techniques has emerged as a powerful tool in the identification of medicinal plants. Husin et al. (2018) demonstrate the effectiveness of using internal transcribed spacers (ITS2) as DNA barcodes for taxonomic classification, which can complement morphological and chemical analyses. This method enhances the reliability of plant identification, particularly in cases where morphological characteristics may be insufficient for accurate classification. The combination of molecular techniques with traditional methods provides a robust framework for ensuring the authenticity of medicinal plants in herbal medicine. Moreover, the selection of appropriate chemical markers is essential for quality control in herbal medicine. Bensoussan et al. (2015) discuss the development of criteria for choosing chemical markers that can ensure the consistency and safety of herbal products. This aspect is critical, as the therapeutic efficacy of medicinal plants often hinges on their chemical composition. By employing AI-driven methodologies to analyze chemical markers, researchers can establish standardized protocols for quality assurance in herbal medicine, thereby enhancing consumer trust and safety. In summary, the methodologies for AI-driven identification of medicinal plant species encompass a multifaceted approach that integrates morphological analysis, chemical profiling, and molecular techniques. The use of advanced ML algorithms and DL models significantly enhances the accuracy of plant identification, while the incorporation of chemical markers and DNA barcoding provides a comprehensive understanding of plant identity and quality. These methodologies not only advance the field of botany but also contribute to the sustainable use of medicinal plants in healthcare.
3.2.1.1. Image recognition techniques. AI-based image recognition systems utilize CNNs to analyze images of plant leaves and flowers. These systems are trained on large datasets of labeled images, allowing them to learn the distinguishing features of various species. For instance, studies have demonstrated that CNNs can achieve high accuracy in classifying plant species based on leaf morphology, with some models reporting accuracy rates exceeding 90% (Akhmetova et al., 2018; Hernández-Salón, 2023). The ability to quickly process and analyze visual data makes AI a powerful tool for botanists and ethnobotanists.
AI, or rather computer vision, is considered a powerful tool that can process and analyze images and identify and classify the desired objects. But why and how is the model able to achieve such results? For this purpose, when training such effective models on images, there is a multilevel process involved.
First, a large set of labeled, annotated images, in this case medicinal plants, is defined. These can be obtained from public sources or from collected data in field observations; however, an important point to consider is the diversity and balance of the data. The images should be taken in different conditions, from different angles, with different lighting conditions and backgrounds that the model is robust and can be trained on all possible cases. It is also common to use augmentation method in addition to the existing dataset. Augmentation is a method of increasing the volume of data by random rotations, reflections, and adding brightness and contrast to existing images. This technique and data processing help to increase the robustness of the model, reducing the probability of overfitting.
Second, all images must be preprocessed as many computer vision models require images to be of the same format. This includes resizing the images to a standard and common size, also normalizing the pixel intensity which helps to increase the convergence rate of training.
Third, the processed data is split into three main sets: a training set on which the model will be trained, a validation set with which the models and optimized hyperparameters will be evaluated, and a test set which is essentially unseen data for final evaluation.
Fourth, these images are fed as input to the computer vision model, which basically consists of CNN. At this stage, the model will extract from the images important features such as shape, texture, color, and structural features of leaves and flowers that can characterize specific plant species.
Fifth, the extracted features pass through several layers of the neural network where they are analyzed and transformed into a linear feature space suitable for classification.
Sixth, after the feature extraction stage, the model makes a final decision on whether an object belongs to a particular class (a particular species). Several final full link layers are used in the process, and the final classification is done using an activation function (e.g., softmax) that returns the probability of belonging to each class. By doing so, not only can the most likely plant species be selected, but also the confidence of the model in its decision can be assessed.
In the end, the results are compared and contrasted with the true labels with commonly used metrics such as accuracy, F1-measure, completeness, and precision. Explainability methods (e.g., Grad-CAM, LIME) are also utilized so that the regions of the image that led to the final model solution can be visually interpreted. This is especially important in interdisciplinary collaborations: botanists and AI experts can jointly analyze whether the model correctly extracts and uses biologically relevant features.Thus, the integration of the steps of data collection, careful preprocessing, model training using state-of-the-art CNN architectures and interpretation methods allows the development of powerful tools for automated classification of medicinal plants, which greatly facilitates species identification and improves the quality of research in ethnobotany and pharmaceutical sciences.
3.2.1.2. Chemical marker analysis. In addition to morphological traits, chemical markers play a vital role in the identification of medicinal plants. Secondary metabolites, such as alkaloids, flavonoids, and terpenoids, are often responsible for the therapeutic properties of plants. AI can assist in analyzing chemical profiles through techniques such as gas chromatography-mass spectrometry (GC-MS) and HPLC. By integrating chemical data with morphological characteristics, researchers can enhance the accuracy of species identification and better understand the medicinal properties of plants (Ramli et al., 2021; Rambey and Onrizal, 2022).
3.2.1.3. Data integration and machine learning. The integration of morphological and chemical data into ML models allows for a more comprehensive approach to plant identification. For example, researchers have employed RF algorithms and SVMs to classify plant species based on combined datasets of leaf images and chemical profiles. This multifaceted approach has been shown to improve classification accuracy compared to using morphological or chemical data alone (Akhilraj, 2023; Rambey et al., 2022).
3.2.2. Applications and benefits of AI in plant identification
The application of AI in the identification of medicinal plant species has gained significant traction in recent years, driven by advancements in ML, image processing, and chemical analysis techniques. These methodologies not only enhance the accuracy of plant identification but also contribute to the conservation and sustainable use of medicinal plants, which are vital for healthcare and traditional medicine (Prasanth, 2018).
One of the most promising applications of AI in medicinal plant identification is through DL algorithms, particularly CNNs. Goyal (2023) demonstrates the efficacy of an optimized CNN model, HerbaVisionNet, which achieved an impressive accuracy rate of 99% in classifying 30 different medicinal plant species based on high-resolution images of their leaves and flowers. This level of precision is crucial for ensuring the correct identification of plants, which directly impacts the efficacy and safety of herbal remedies. Similarly, the study by Yue et al. (2021) highlights the superiority of DL techniques combined with two-dimensional correlation spectroscopy (2DCOS) for identifying medicinal plants, showcasing how these technologies can streamline the identification process without the need for complex spectral preprocessing (Elmosallamy et al., 2022).
In addition to the image-based identification, AI methodologies also incorporate chemical markers for a more comprehensive analysis. For instance, DNA barcoding has emerged as a valuable tool for the molecular identification of medicinal plants. Nabil et al. emphasize the importance of DNA barcoding in ensuring the authenticity and therapeutic value of medicinal plants, particularly in combating issues related to illegal trade and adulteration. This molecular approach, when combined with AI techniques, can significantly enhance the reliability of plant identification, ensuring that herbal products are safe and effective for consumer use (Yue et al., 2021).
Moreover, the integration of AI in the identification process not only improves accuracy but also facilitates rapid assessments, which are essential in both research and commercial contexts. The work by Ydyrys (2023) provides an inventory of medicinal plants, underscoring the need for accurate identification to prevent poisoning and promote public health. This highlights the broader implications of AI-driven identification methodologies, as they contribute to the conservation of biodiversity and the sustainable use of plant resources.
The benefits of AI extend beyond identification; they also encompass the potential for enhanced research and development in pharmacognosy. Prasanth (2018) discusses the importance of pharmacognostic studies in the standardization of medicinal plants, indicating that while modern techniques are available, the integration of AI can further streamline these processes. By automating the identification and classification of medicinal plants, researchers can focus on exploring their phytochemical properties and therapeutic applications more efficiently.
Furthermore, the application of AI in medicinal plant identification is not limited to academic research; it also has practical implications for the pharmaceutical industry. The ability to quickly and accurately identify plant species can accelerate the discovery of new drugs and herbal formulations, as highlighted by Wong (2023), who discussed the transformative potential of AI in pharmacy practice. This integration of AI can lead to more effective medication management and improved patient outcomes.
3.2.2.1. Ethnobotanical research. AI-driven identification methods facilitate ethnobotanical studies by enabling researchers to catalog and analyze the medicinal plants used by indigenous communities. For instance, studies have documented the medicinal plants utilized by various ethnic groups, highlighting the importance of preserving traditional knowledge alongside biodiversity (Tefera and Yihune, 2019; Adedeji et al., 2018). AI can streamline the process of identifying and documenting these species, ensuring that valuable ethnobotanical knowledge is not lost.
3.2.2.2. Conservation efforts. The identification of medicinal plant species is crucial for conservation initiatives aimed at protecting endangered species and their habitats. By utilizing AI technologies, conservationists can monitor plant populations and assess the impact of environmental changes on medicinal plants. This proactive approach can help mitigate the risks associated with habitat loss and overharvesting (Rahmawaty et al., 2019; Ebu et al., 2021).
3.2.2.3. Sustainable use of medicinal plants. AI technologies can also support the sustainable use of medicinal plants by providing accurate identification tools for herbal practitioners and consumers. This can help prevent the misuse of plant species and promote the conservation of valuable medicinal resources. For example, accurate identification can reduce the risk of using toxic or endangered species in traditional medicine (Ydyrys et al., 2023; Cui et al., 2020).
3.2.2.4. Challenges and future directions. Despite the promising advancements in AI for plant identification, several challenges remain. The need for large, high-quality datasets for training AI models is critical, as is the potential for bias in model predictions based on the training data used. Future research should focus on developing more inclusive datasets that represent the full diversity of medicinal plants and their morphological and chemical variations (Luo et al., 2019; Xie et al., 2023). Additionally, interdisciplinary collaboration between botanists, chemists, and data scientists will be essential for advancing AI applications in this field.
In conclusion, the applications of AI in the identification of medicinal plant species are multifaceted, encompassing image analysis, chemical profiling, and molecular techniques. These methodologies not only enhance the accuracy and efficiency of plant identification but also contribute to the conservation of medicinal biodiversity and the development of safe and effective herbal remedies. As AI technology continues to evolve, its integration into the field of botany and pharmacognosy promises to revolutionize the way we identify and utilize medicinal plants.
The integration of AI in identifying medicinal plant species based on leaf patterns, flowers, and chemical markers represents a significant advancement in botanical research. By enhancing the accuracy and efficiency of plant identification, AI technologies can support ethnobotanical studies, conservation efforts, and the sustainable use of medicinal plants. Continued research and collaboration in this area will undoubtedly lead to more refined methodologies and broader applications, ultimately contributing to the preservation of plant biodiversity and traditional medicinal knowledge.
3.3. Classify plants automatically using large botanical databases
The classification of plants has traditionally relied on morphological characteristics, which can be subjective and labor-intensive. However, the integration of AI technologies, particularly ML and DL algorithms, has transformed the classification process. AI can analyze vast datasets from botanical databases, including images, DNA sequences, and ecological data, to classify plant species with high accuracy. This capability is crucial for addressing global biodiversity challenges and enhancing our understanding of plant taxonomy.
The classification of plants using large botanical databases and AI has emerged as a transformative approach in botany, ecology, and conservation. This integration of technology allows for rapid and accurate identification of plant species, which is essential for biodiversity assessments, ecological studies, and sustainable management of plant resources.
One of the primary methodologies for automated plant classification involves the use of DL techniques, particularly CNNs. These models have demonstrated remarkable success in identifying plant species from images of leaves and flowers. For instance, Singh (2023) highlights the effectiveness of CNNs in classifying plants at various taxonomic levels, enabling users to identify species through simple photographs taken with mobile devices. This accessibility is crucial for citizen science initiatives and ecological surveys where nonexperts can contribute valuable data without requiring extensive botanical knowledge.
The importance of large botanical databases cannot be overstated, as these provide the foundational data necessary for training AI models. Wäldchen and Mäder (2017) emphasize that the most commonly studied plant organs for identification are leaves, due to their availability throughout the year and ease of imaging. This focus simplifies the data acquisition process and enhances the reliability of the classification models. Furthermore, the integration of diverse datasets from various sources, such as herbarium specimens and citizen science contributions, enriches the training data, leading to improved model performance (Chulif et al., 2022).
Automated plant identification systems also face challenges, particularly due to the vast number of species that exist globally. In Europe alone, there are over 20,000 vascular plant species, making it imperative for AI systems to manage a large number of visual classes effectively (Pärtel et al., 2021). Jones (2020) discusses the limitations of the current automated identification applications, noting that while they can provide high confidence in many cases, they may struggle with species that require specific seasonal or microscopic characteristics for accurate identification. This highlights the need for continuous improvement in AI algorithms and the incorporation of additional features, such as chemical markers or habitat data, to enhance classification accuracy.
The application of AI in plant classification extends beyond mere identification, as it also plays a crucial role in conservation efforts. By automating the identification process, researchers can conduct large-scale biodiversity assessments more efficiently. For instance, the work by August et al. (2020) illustrates how AI classifiers can analyze social media imagery to unlock biodiversity data, facilitating the monitoring of plant species in various ecosystems. This capability is vital for tracking changes in plant populations and informing conservation strategies.
Moreover, the development of XAI frameworks is essential in fostering trust in the automated classification systems. Ghosal et al. (2018) propose a deep machine vision framework that not only identifies plant stress but also provides interpretable insights into the classification process. Such transparency is crucial for researchers and practitioners who rely on these technologies for decision-making in conservation and agriculture.
The classification of plants using large botanical databases and AI represents a significant advancement in the field of botany. The integration of DL techniques with extensive datasets facilitates rapid and accurate plant identification, contributing to biodiversity conservation and ecological research. As technology continues to evolve, the potential for AI to enhance our understanding of plant diversity and support sustainable management practices will only grow.
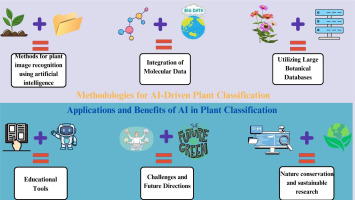
3.3.1. Methodologies for AI-driven plant classification
The classification of medicinal plants using AI has emerged as a transformative approach, leveraging large botanical databases to enhance accuracy and efficiency in plant identification. Figure 4 shows various methodologies employed in the automatic classification of medicinal plants, focusing on image analysis, DL, and the integration of botanical data (Figure 4).
One of the foundational methodologies in this domain is the use of DL techniques, particularly CNNs, for image classification. Research by Ghosh et al. (2023) emphasizes the effectiveness of hybrid transfer learning techniques in identifying medicinal plants, demonstrating that DL approaches significantly outperform traditional ML methods in terms of accuracy and reliability. The study highlights the importance of utilizing large datasets, such as the LeafSnap dataset, to train models capable of recognizing intricate features of plant leaves, which are crucial for accurate identification (Manjunath et al., 2024). Furthermore, Pushpanathan et al. (2022) discuss the development of MYLPHerb-1, a dataset specifically designed for classifying Malaysian local perennial herbs, underscoring the necessity for real-world data to enhance model performance in uncontrolled environments.
The integration of multiple descriptors and feature extraction techniques is another critical aspect of the automatic classification systems. For instance, the work by Alibek et al. (2024) presents a hierarchical approach to plant leaf classification that utilizes various descriptors, enhancing the robustness of the classification process. This methodology allows for a more nuanced understanding of plant morphology, facilitating the identification of species based on a combination of leaf shape, texture, and color. Additionally, the use of advanced optimization techniques, such as particle swarm optimization (PSO) combined with fuzzy c-means clustering, has been proposed by Kumar et al. (2019) to improve segmentation and classification accuracy in leaf disease prediction, which can also be adapted for medicinal plant classification.
Moreover, the application of ensemble learning methods, as explored by Hamrouni et al. (2018), demonstrates the potential of combining multiple classifiers to improve identification accuracy. By utilizing classifiers such as RF and SVMs in parallel, the study shows that a hybrid approach can effectively leverage the strengths of different algorithms to enhance classification performance (Hamrouni et al., 2018). This methodology is particularly beneficial in scenarios where plant features may vary significantly due to environmental factors.
The role of large botanical databases cannot be overstated in the context of AI-driven classification. Wäldchen et al. (2018) highlight the necessity of accessible and up-to-date databases for automating species identification, which is crucial for biodiversity conservation efforts. The integration of comprehensive botanical data with AI algorithms enables researchers and practitioners to quickly and accurately identify medicinal plants, thereby supporting sustainable practices and reducing the risk of misidentification.
Furthermore, the advancements in AI methodologies have significant implications for the pharmaceutical industry. The ability to rapidly classify and identify medicinal plants can accelerate the discovery of new herbal remedies and enhance the quality control processes for existing products. As noted by Attallah and Sharkas (2021), the integration of AI in classification tasks can lead to more efficient workflows and improved outcomes in various applications, including drug development. However, it is important to note that the reference provided discusses skin cancer classification rather than medicinal plant classification, which may not directly support the claim made (Attallah & Sharkas, 2021).
In conclusion, the methodologies for classifying medicinal plants automatically using AI are diverse and multifaceted. The combination of DL techniques, feature extraction methods, and the utilization of large botanical databases has revolutionized the field of plant identification. As these technologies continue to evolve, they hold the promise of enhancing our understanding of medicinal plants and supporting their sustainable use in healthcare.
3.3.1.1. Image recognition techniques. One of the most significant applications of AI in plant classification is through image recognition. CNNs are widely used to analyze images of plant leaves, flowers, and fruits. These models are trained on large datasets, enabling them to learn distinguishing features of various species. For instance, studies have shown that CNNs can achieve classification accuracies exceeding 90% when identifying plant species based on leaf morphology. The ability to process and analyze images rapidly allows for efficient classification, even in large-scale biodiversity assessments.
3.3.1.2. Integration of molecular data. In addition to morphological data, molecular techniques such as DNA barcoding and sequencing play a crucial role in plant classification. AI can analyze genetic data alongside morphological traits to enhance classification accuracy. For example, the integration of DNA markers with phenotypic data has been shown to provide a more comprehensive understanding of plant diversity and evolutionary relationships. This multifaceted approach allows researchers to classify plants more reliably, especially in cases where morphological characteristics may be ambiguous.
3.3.1.3. Utilizing large botanical databases. The availability of large botanical databases, such as herbarium collections and genomic databases, has significantly enhanced the potential for AI-driven classification. Platforms like eHerbarium provide access to extensive collections of plant images and associated metadata, which can be leveraged by AI algorithms to improve classification outcomes. These databases not only facilitate the training of AI models but also enable researchers to validate their findings against a broader spectrum of data.
3.3.2. Applications and benefits of AI in plant classification
The methodologies for the application of AI in the classification of medicinal plants have become increasingly sophisticated, leveraging advancements in ML, image processing, and data analytics. These methodologies not only enhance the accuracy of plant identification but also facilitate the exploration of the therapeutic potential of various species, thereby contributing to the fields of pharmacognosy and herbal medicine. One of the primary methodologies employed in medicinal plant classification is the use of DL techniques, particularly CNNs. For instance, Goyal (2023) developed HerbaVisionNet, an optimized CNN model that achieved a remarkable accuracy of 99% in classifying 30 different medicinal plant species based on leaf and flower images. This high level of precision is critical for ensuring the correct identification of medicinal plants, which directly influences their therapeutic efficacy. Similarly, Sapna et al. (2022) utilized a feedforward neural network structure combined with bacterial foraging optimization to enhance the classification accuracy of medicinal plant leaves, demonstrating the effectiveness of integrating optimization techniques with DL. These approaches underscore the potential of AI to automate and streamline the classification process, making it more efficient and reliable. In addition to image-based classification, methodologies that incorporate chemical markers and molecular data are also gaining traction. The work by Islam (2024) highlights the use of PSO in cascaded networks for classifying medicinal plants, focusing on both leaf disease detection and species classification. This dual approach not only aids in identifying plant species but also addresses the critical issue of plant health, which is essential for maintaining the quality of herbal products. Furthermore, the integration of DNA barcoding techniques enhances the reliability of plant identification by providing a molecular basis for classification, ensuring that medicinal plants are accurately authenticated. Moreover, the development of mobile applications for medicinal plant recognition exemplifies the practical applications of AI methodologies. Sugiarto et al. (2023) demonstrated how CNNs can be employed in mobile applications to recognize medicinal plants from leaf images, providing users with information about the plants’ medicinal properties and efficacy. This accessibility not only empowers individuals to identify and utilize medicinal plants but also promotes the conservation of biodiversity by encouraging sustainable harvesting practices. The benefits of AI in medicinal plant classification extend beyond mere identification, as they also encompass the potential for enhanced research and development in herbal medicine. The systematic review by Zinchenko et al. (2022) emphasize the importance of postmarketing surveillance for software as a medical device (SaMD) based on AI technologies, highlighting the need for robust methodologies to ensure the efficacy and safety of AI applications in healthcare. This is particularly relevant in the context of medicinal plants, where accurate classification and validation of therapeutic properties are crucial for consumer safety and regulatory compliance. Furthermore, the integration of AI methodologies in medicinal plant classification can lead to significant advancements in drug discovery and development. By accurately identifying and classifying medicinal plants, researchers can explore their phytochemical properties and therapeutic applications more efficiently. This is particularly important in the context of increasing interest in natural products as alternatives to synthetic pharmaceuticals, as highlighted by the growing demand for herbal remedies in the global market (Vozhehova, 2023). In conclusion, the methodologies for AI-driven classification of medicinal plants encompass a range of advanced techniques, including DL, chemical analysis, and mobile applications. These methodologies enhance the accuracy and efficiency of plant identification, facilitate research in pharmacognosy, and promote the sustainable use of medicinal resources.
3.3.2.1. Educational tools. AI-driven plant classification systems can also serve as valuable educational tools. Mobile applications that utilize AI for plant identification can engage students and the public in botanical studies, fostering a greater appreciation for plant diversity and conservation (Khan et al., 2024). These tools can enhance outdoor learning experiences, helping users identify and understand the ecological significance of local flora.
3.3.2.2. Challenges and future directions. Despite the advancements in AI-driven plant classification, several challenges remain. The need for high-quality, annotated datasets is critical for training effective AI models. Additionally, there is a risk of bias in AI predictions based on the training data used, which can affect classification accuracy (Aliyu, 2024). Future research should focus on developing more inclusive datasets that represent the full diversity of plant species and their morphological variations. Moreover, interdisciplinary collaboration among botanists, data scientists, and conservationists will be essential for advancing AI applications in this field.
The integration of AI in the automatic classification of plants using large botanical databases represents a significant advancement in botanical research. By enhancing the accuracy and efficiency of plant classification, AI technologies can support biodiversity conservation, improve agricultural practices, and serve as educational tools. Continued research and collaboration in this area will undoubtedly lead to more refined methodologies and broader applications, ultimately contributing to the preservation of plant biodiversity and the advancement of botanical sciences.
3.4. Predictive modeling of bioactivity and phytochemical profiling
ML models can be trained on existing datasets to predict the bioactivity of compounds derived from medicinal plants. Techniques such as quantitative structure-activity relationship (QSAR) modeling allow researchers to estimate the efficacy of new compounds, significantly reducing the time and resources required for experimental validation.
AI can enhance the analysis of complex phytochemical profiles through techniques such as chemoinformatics and metabolomics. By employing clustering algorithms and pattern recognition, researchers can identify and categorize compounds based on their chemical structures and biological activities, leading to the discovery of novel therapeutic agents.
The integration of AI in phytochemical analysis has significantly advanced medicinal plant research, enhancing the identification and characterization of bioactive compounds. Phytochemicals, the biologically active compounds found in plants, play a crucial role in the therapeutic efficacy of medicinal plants. AI technologies, particularly ML and data mining, have been employed to analyze complex phytochemical data, enabling researchers to uncover relationships between chemical constituents and their medicinal properties more efficiently than traditional methods (Mohanraj et al., 2018; Vivek-Ananth et al., 2023). One of the key applications of AI in phytochemical analysis is in the automated identification of phytochemical profiles. For instance, GC-MS is a widely used technique for analyzing the chemical composition of plant extracts. AI algorithms can process the vast amounts of data generated by GC-MS, identifying and quantifying phytochemicals with high accuracy (Olivia et al., 2021; Purushothaman et al., 2023). This capability is particularly beneficial in screening large numbers of medicinal plants for their potential therapeutic applications, as it allows for rapid analysis and comparison of phytochemical profiles across different species (Sahu et al., 2019). Moreover, AI can enhance the predictive modeling of phytochemical activity, linking specific compounds to their biological effects. For example, studies have shown that certain phytochemicals, such as flavonoids and phenolic compounds, exhibit antioxidant properties that contribute to their medicinal effects (Haouari et al., 2021; Manikandan, 2022). By employing ML models, researchers can predict the biological activity of unknown compounds based on their structural features, thus accelerating the drug discovery process (Idamansyah, 2021). This predictive capability is essential for identifying new therapeutic agents derived from medicinal plants, as it allows for the prioritization of compounds for further investigation. The use of AI also facilitates the integration of ethnobotanical knowledge with phytochemical data, providing a holistic approach to medicinal plant research. Ethnobotanical studies often highlight the traditional uses of plants, which can guide researchers in selecting species for phytochemical analysis (Ydyrys et al., 2024; Te et al., 2020). By combining ethnobotanical insights with AI-driven data analysis, researchers can better understand the relationship between traditional uses and the pharmacological potential of medicinal plants, leading to more informed selection of candidates for drug development (Timsina, 2023). Despite the promising advancements, challenges remain in the application of AI in phytochemical analysis. The quality and diversity of data are critical for the success of AI models, as biased or incomplete datasets can lead to inaccurate predictions (Mattummal et al., 2019; Rao et al., 2023). Additionally, the interpretability of AI models is a concern, as understanding the rationale behind AI-driven predictions is essential for validating results in the context of medicinal plant research (Challen et al., 2019). Addressing these challenges will be crucial for maximizing the potential of AI in advancing phytochemical analysis and medicinal plant research. In conclusion, the incorporation of AI in phytochemical analysis represents a significant advancement in medicinal plant research. By enhancing the identification and characterization of bioactive compounds, improving predictive modeling, and integrating traditional knowledge with scientific analysis, AI technologies are poised to revolutionize the field. Continued research and development in this area will likely yield new insights and therapeutic applications derived from the rich biodiversity of medicinal plants.
The predictive modeling of bioactivity and phytochemical profiling using AI has emerged as a transformative approach in drug discovery and natural product research. This methodology leverages ML algorithms and computational techniques to analyze complex datasets, enabling researchers to predict the biological activity of compounds and their potential therapeutic applications. The integration of bioactivity data with phytochemical profiles enhances the understanding of the relationship between chemical structure and biological function, facilitating the identification of promising candidates for further development. One of the foundational methodologies in predictive modeling is the QSAR approach, which correlates the chemical structure of compounds with their biological activity, allowing for the prediction of the activity of new compounds based on their structural features. For instance, Xu et al. (2020) demonstrated that combining bioactivity data with chemical descriptors significantly improved the prediction of human organ toxicity, highlighting the effectiveness of QSAR models in assessing the safety and efficacy of compounds. This approach is particularly valuable in the early stages of drug discovery, where it can guide the selection of compounds for further testing. ML techniques have further advanced the capabilities of predictive modeling in bioactivity assessment. Walker and Clardy (2021) developed a ML bioinformatics method that predicts biological activity from biosynthetic gene clusters (BGCs). By training classifiers on datasets of known BGCs and their associated activities, they were able to create models that can predict the bioactivity of natural products based on their genetic information. This innovative approach not only streamlines the identification of bioactive compounds but also emphasizes the importance of genetic data in understanding the biosynthesis of natural products. Moreover, the use of advanced algorithms such as RFs and SVMs has been shown to enhance predictive accuracy. Egieyeh et al. (2018) utilized ML classifiers to predict antimalarial bioactivity from a library of natural products, demonstrating that RF models outperformed other classifiers in terms of predictive reliability. This finding underscores the potential of ML to analyze complex biological data and identify compounds with significant therapeutic potential. In addition to traditional QSAR models, recent advancements have introduced multimodal approaches that integrate various types of data, including chemical, biological, and molecular information. The research by Yip (2023) on pretraining multimodal bioactivity profiles illustrates how combining different data modalities can enhance the prediction of small molecule properties. This approach allows for a more comprehensive understanding of how different factors contribute to bioactivity, paving the way for more accurate predictions.
It is now becoming a common practice to combine multimodal data to improve the accuracy and reliability of classification. In this method, information is taken from multiple modalities (e.g., leaf photographs, chemical composition, and genetic information) and merged using multimodal learning algorithms.
There are two main ways of fusing such data with multimodal learning:
The first is late fusion. In this approach, you take a pretrained model to handle each individual data type (e.g., one for images and one for chemical or genetic data), with the models generating their own predictions. In the last step, the predictions are combined (e.g., by averaging the probabilities or using a more theoretically sophisticated ensemble learning mechanism) to solve the problem. Late pooling allows one to use separate optimally tuned models for each modality, and therefore reduces the risk that noise from one modality would mutually influence the other.
The second, the ensemble methods, is the moment of combining features obtained from different sources in the previous stages of data processing. The features from each channel (i.e., images, chemical data, genetic sequences) are combined into a single representation to train the final classifier. This approach achieves synergy by jointly analyzing different domains of plant information, and improves the overall classification accuracy.
The application of multimodal methods is particularly valuable in tasks where each modality carries a unique bioinformational load. For example, images can provide detailed morphological information, chemical profiles can provide information on bioactive compound composition, and genetic data can provide information on phylogeny and genetic affiliation. Combining these data can greatly improve the accuracy and reliability of models, as evidenced by the current research in automating medicinal plant identification.
Furthermore, the application of conformal prediction models has gained traction in the field of bioactivity prediction. Lomana et al. (2021) highlighted the utility of conformal prediction in enhancing the reliability of bioactivity predictions by providing probabilistic estimates of activity based on model outputs. This methodology not only improves the interpretability of predictions but also helps in assessing the uncertainty associated with bioactivity estimates, which is crucial for making informed decisions in drug development. The integration of high throughput screening data with predictive modeling is another significant advancement in this field. Gao et al. (2020) emphasized the importance of accurately predicting compound bioactivities for virtual screening processes, which are essential for identifying lead compounds from large chemical libraries. By employing ML algorithms to analyze screening data, researchers can prioritize compounds for experimental validation, thereby accelerating the drug discovery process. In conclusion, the methodologies for predictive modeling of bioactivity and phytochemical profiling using AI are diverse and rapidly evolving. The integration of QSAR models, ML techniques, multimodal data approaches, and conformal prediction frameworks enhances the accuracy and reliability of bioactivity predictions. These methodologies not only facilitate the identification of promising therapeutic candidates but also contribute to a deeper understanding of the relationship between chemical structure and biological activity. As AI technologies continue to advance, their application in predictive modeling will play a crucial role in the future of drug discovery and natural product research (Figure 5).
The methodologies for predictive modeling of bioactivity and phytochemical profiling using AI have become increasingly relevant in the field of pharmacognosy and natural product research. These methodologies leverage advanced computational techniques to analyze the relationships between the chemical composition of plant extracts and their biological activities, thereby facilitating the discovery of novel therapeutic agents. One of the foundational methodologies in this domain is the application of ML algorithms to predict the bioactivity of phytochemicals. El-Attar et al. conducted a comparative study of two ML models, the multiclass neural network (MNN) and CNN, to predict the biological activity of essential oil-producing plants from Egypt. Their results demonstrated that the CNN model outperformed the MNN, achieving a prediction accuracy of 98.13% compared to 81.88% (Mihaylova, 2024). This highlights the efficacy of DL approaches in capturing complex patterns in biological data, which is essential for accurate bioactivity predictions. In addition to DL, the integration of chemometric models, such as composition-activity relationships (CARs), has been explored to enhance predictive modeling. Yabuuchi (2024) proposed a chemometric model that accounts for the relationships between the chemical compositions of plant extracts and their bioactivity. This approach allows researchers to systematically analyze how variations in chemical constituents influence biological activity, thereby guiding the selection of plant species for further investigation. Phytochemical profiling is another critical aspect of predictive modeling. Techniques such as GC-MS are widely used to identify and quantify bioactive compounds in plant extracts. Desai et al. (2025) utilized GC-MS to analyze the bioactive compounds in Curcuma longa leaves, demonstrating the technique’s effectiveness in providing detailed phytochemical profiles. The data obtained from such analyses can be integrated into AI models to improve the accuracy of bioactivity predictions by correlating specific compounds with their therapeutic effects. Moreover, the use of endophytic fungi in medicinal plants has emerged as a promising area of research. Xingyuan et al. (2022) highlighted the potential of bioactive metabolites derived from endophytic fungi, which can be isolated and studied for their therapeutic bioactivity. By employing AI methodologies to analyze the chemical profiles of these metabolites, researchers can identify novel compounds with potential medicinal applications. The role of phylogenetics in predicting the presence of bioactive compounds is also noteworthy. Johnson-Fulton and Watson (2018) emphasized that closely related plant species often share medicinally active compounds, suggesting that phylogenetic frameworks can be utilized to target species for further research. This approach can be enhanced by AI algorithms that analyze genetic data alongside chemical profiles to predict the presence of bioactive compounds in uninvestigated species. Furthermore, the optimization of abiotic stress conditions has been shown to influence the production of secondary metabolites in plants. Hurmat et al. (2020) reviewed how various abiotic stresses can enhance the levels of bioactive compounds, thereby increasing the bioactivity of medicinal plants. AI-driven predictive models can be employed to simulate different environmental conditions and their effects on metabolite production, aiding in the selection of optimal growth conditions for medicinal plants. In conclusion, the methodologies for predictive modeling of bioactivity and phytochemical profiling using AI encompass a variety of advanced techniques, including ML, chemometric modeling, and phytochemical analysis. These methodologies not only enhance the understanding of the relationships between plant chemistry and biological activity but also facilitate the discovery of novel therapeutic agents. As AI technology continues to advance, its integration into the field of pharmacognosy promises to revolutionize the identification and development of medicinal plants.
3.4.1. Genomic and transcriptomic analysis
AI-driven bioinformatics tools can analyze genomic and transcriptomic data from medicinal plants, facilitating the identification of genes associated with the biosynthesis of bioactive compounds. This can lead to the development of genetically modified plants with enhanced medicinal properties or the identification of plant varieties with superior therapeutic potential.
The integration of genomic and transcriptomic analyses with AI has revolutionized the study of medicinal plants, enabling researchers to uncover the complex relationships between genetic information and the bioactive compounds these plants produce. This approach enhances our understanding of the biosynthesis of medicinal compounds and facilitates the identification of key genes involved in their production, ultimately aiding in the development of new therapeutic agents.
One of the foundational methodologies in this field is the use of next-generation sequencing (NGS) technologies, which have significantly advanced the genomic and transcriptomic analysis of medicinal plants. Chaudhary and Sharma (2016) highlight that NGS allows for the identification of key genes responsible for the biosynthesis of bioactive phytocompounds, providing a comprehensive understanding of the metabolic pathways involved. This is particularly important for medicinal plants, where the complexity of their genomes often poses challenges for traditional sequencing methods. For example, the genome sequencing of Tinospora cordifolia revealed several compounds responsible for its medicinal properties, such as anti-inflammatory and antioxidant activities, including tinosporaside and phenolic compounds (Mahajan et al., 2021). By employing AI algorithms to analyze the vast amounts of data generated by NGS, researchers can identify patterns and correlations that may not be immediately apparent through manual analysis.
Ribonucleic acid sequencing (RNA-seq) has emerged as a powerful tool for transcriptomic analysis, allowing for the examination of gene expression profiles in response to various environmental conditions. For instance, Rastogi et al. (2019) conducted a metabolomic analysis of Ocimum sanctum under abiotic stress conditions, revealing significant changes in gene expression related to secondary metabolite production. This type of analysis can be enhanced through AI-driven predictive modeling, which can forecast how changes in environmental factors may influence the biosynthesis of medicinal compounds. Additionally, the integration of transcriptomic data with metabolomic profiles, as demonstrated by Lang et al. (2019) in their study of Michelia maudiae, provides insights into the metabolic pathways that govern the production of bioactive compounds. AI can facilitate this integration by employing ML algorithms to correlate gene expression data with metabolite profiles, thereby identifying key regulatory genes.
Furthermore, the application of AI in the analysis of transcriptomic data can lead to the discovery of novel splice variants and alternative splicing events that contribute to the diversity of bioactive compounds in medicinal plants. Xu et al. (2015) utilized a combination of sequencing platforms to obtain full-length transcriptomes of Salvia miltiorrhiza, uncovering important insights into the biosynthesis of tanshinones, which are known for their therapeutic properties. AI algorithms can analyze these complex datasets to identify patterns in gene expression that are associated with specific metabolic outcomes, thereby enhancing our understanding of how different genetic factors contribute to the medicinal properties of plants.
The use of AI also extends to the development of genomic databases that facilitate the exploration of medicinal plant genomes and transcriptomes. For example, the establishment of databases like MagnoliidsGDB allows researchers to access a wealth of genomic and transcriptomic data, enabling comparative analyses across species (Chen, 2024). AI-driven tools can assist in the annotation of these genomes and transcriptomes, improving the accuracy and efficiency of data interpretation. This is particularly important for nonmodel organisms, where genomic resources may be limited.
Moreover, the application of AI in genomic and transcriptomic analyses can significantly enhance the efficiency of bioprospecting efforts aimed at discovering new medicinal compounds. By utilizing ML algorithms to analyze existing genomic and transcriptomic data, researchers can prioritize species for further investigation based on their predicted bioactivity. This approach not only accelerates the discovery process but also ensures that resources are allocated effectively.
In conclusion, the methodologies for genomic and transcriptomic analysis of medicinal plants, when combined with AI, offer powerful tools for understanding the genetic basis of bioactive compound production. The integration of NGS technologies, RNA-seq, and AI-driven data analysis enables researchers to uncover the complex interactions between genes and metabolites, paving the way for the discovery of new therapeutic agents. As these technologies continue to evolve, they hold great promise for advancing the field of medicinal plant research and enhancing the development of phytomedicines.
The methodologies for genomic and transcriptomic analysis of medicinal plants, particularly when integrated with AI, have significantly advanced our understanding of the genetic basis of bioactive compound production. These methodologies leverage high throughput sequencing technologies, bioinformatics tools, and ML algorithms to analyze complex datasets, facilitating the identification of key genes and metabolic pathways involved in the biosynthesis of medicinal compounds (Figure 6).
Figure 6
The methodologies for genomic and transcriptomic analysis of medicinal plants, particularly when integrated with AI.
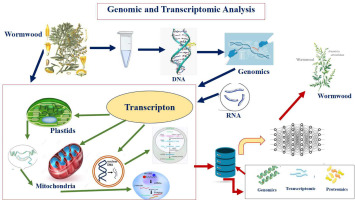
3.4.1.1. Genomic analysis. Genomic analysis of medicinal plants typically begins with the application of NGS technologies, which allow for comprehensive genome assembly and annotation. For instance, Zhao et al. (2019) provided insights into the evolution of wogonin biosynthesis in Scutellaria baicalensis through the sequencing of its reference genome, revealing the genetic basis for the production of flavonoids, which are critical for the plant’s medicinal properties. This type of genomic analysis is essential for identifying BGCs responsible for the production of specific bioactive compounds. Similarly, Liu et al. (2021) analyzed the genome of Coptis chinensis, uncovering the diversification of protoberberine-type alkaloids. Their study utilized benchmarking universal single-copy orthologs (BUSCO) analysis to ensure the completeness of the genome assembly, which is crucial for accurate functional annotation. Such detailed genomic insights enable researchers to explore gene duplication events and the evolution of metabolic pathways that contribute to the medicinal properties of these plants.
3.4.1.2. Transcriptomic analysis. Transcriptomic analysis complements genomic studies by providing insights into gene expression patterns under various environmental conditions. RNA-seq is a widely used technique for this purpose, allowing researchers to assess the expression levels of genes involved in the biosynthesis of medicinal compounds. For example, Guo et al. (2021) emphasized the importance of transcriptomics in identifying genes related to the accumulation of medicinal components, noting that gene expression patterns can vary significantly based on growth conditions and developmental stages. This variability underscores the need for comprehensive transcriptomic analyses to identify key regulatory genes.
Moreover, the integration of transcriptomic data with metabolomic profiles can enhance our understanding of the metabolic pathways that govern the production of bioactive compounds. For instance, the study by Kim et al. (2018) on *Panax ginseng* involved the reconstruction of a genome-scale metabolic network, which facilitated the identification of candidate genes associated with the overproduction of ginsenosides, a class of bioactive compounds with significant therapeutic potential. AI algorithms can be employed to analyze these complex datasets, identifying correlations between gene expression and metabolite production.
3.4.1.3. AI integration. The integration of AI into genomic and transcriptomic analyses enhances the ability to process and interpret large-scale biological data. AI algorithms, particularly ML techniques, can identify patterns and predict gene functions based on genomic and transcriptomic data. For example, the application of DL models can facilitate the identification of novel gene functions and regulatory networks involved in the biosynthesis of medicinal compounds (Alrefaei et al., 2022). This approach allows researchers to prioritize candidate genes for further functional validation.
Furthermore, AI-driven databases, such as the Traditional Chinese Medicine Plant Genome Database (TCMPG), provide centralized resources for researchers to access genomic and transcriptomic data related to medicinal plants (Meng et al., 2022). These databases facilitate comparative analyses and enable researchers to explore the genetic diversity of medicinal plants, ultimately aiding in the discovery of new therapeutic agents.
3.4.1.4. Challenges and future directions. Despite the advancements in genomic and transcriptomic analyses, challenges remain in the field. The complexity of plant genomes, particularly in polyploid species, can complicate genome assembly and annotation. Additionally, the integration of diverse datasets from different studies requires standardized protocols and data formats to ensure compatibility and reproducibility.
Future research should focus on enhancing the resolution of genomic and transcriptomic analyses through improved sequencing technologies and AI algorithms. The development of more sophisticated ML models that can integrate multiomics data (genomics, transcriptomics, and metabolomics) should provide deeper insights into the biosynthesis of medicinal compounds and their regulatory mechanisms.
The methodologies for genomic and transcriptomic analysis of medicinal plants, when combined with AI, offer powerful tools for understanding the genetic basis of bioactive compound production. The integration of NGS technologies, RNA-seq, and AI-driven data analysis enables researchers to uncover complex interactions between genes and metabolites, paving the way for the discovery of new therapeutic agents. As these technologies continue to evolve, they hold great promise for advancing the field of medicinal plant research and enhancing the development of phytomedicines.
3.6. Prediction and treatment optimization medicinal plant with AI
The integration of AI in disease prediction and treatment optimization, particularly in the context of medicinal plants, has emerged as a promising frontier in healthcare. This approach leverages advanced computational techniques to analyze complex biological data, enabling the identification of potential therapeutic agents and the optimization of treatment protocols. The synergy between AI and traditional herbal medicine enhances our understanding of the pharmacological properties of medicinal plants and facilitates personalized medicine strategies. AI technologies, particularly ML algorithms, have been instrumental in predicting disease outcomes and tailoring treatments based on individual patient profiles. For instance, AI can analyze large datasets from clinical trials and patient records to identify patterns that correlate with treatment responses. This capability is crucial in the context of herbal medicine, where variability in patient responses to plant-derived compounds can be significant. By employing predictive modeling, researchers should be able to optimize treatment regimens that incorporate medicinal plants, ensuring that patients receive the most effective therapies based on their unique genetic and phenotypic characteristics. Moreover, the application of AI in liquid biopsy techniques has shown promise in early disease detection and monitoring treatment efficacy. Liquid biopsies allow for the analysis of tumor-derived materials from body fluids, providing real-time insights into disease progression and treatment responses. By integrating AI with liquid biopsy data, clinicians can stratify patients more effectively, identifying those who are likely to benefit from specific herbal treatments. This approach enhances the precision of disease management and minimizes the risks associated with ineffective therapies. The role of AI in optimizing treatment protocols not only extends beyond prediction, but it also encompasses the development of personalized medicine approaches. For example, AI algorithms can analyze the chemical profiles of medicinal plants and their interactions with biological targets, facilitating the identification of synergistic combinations that enhance therapeutic efficacy. This is particularly relevant in the treatment of complex diseases such as cancer, where multitargeted therapies derived from medicinal plants can be optimized using AI-driven insights. By understanding the molecular mechanisms underlying the actions of these compounds, healthcare providers can devise more effective treatment strategies that leverage the full potential of herbal medicine. Furthermore, the use of AI in monitoring disease progression and treatment outcomes is gaining traction. ML models can analyze longitudinal patient data to detect subtle changes in health status, allowing for timely interventions. This capability is especially beneficial in chronic disease management, where continuous monitoring is essential for optimizing treatment plans. By integrating AI with telehealth platforms, healthcare providers can ensure that patients using medicinal plants receive ongoing support and adjustments to their treatment regimens as needed. In conclusion, the application of AI in disease prediction and treatment optimization, particularly in the realm of medicinal plants, represents a significant advancement in personalized healthcare. By harnessing the power of AI to analyze complex biological data and optimize treatment protocols, researchers and clinicians can enhance the efficacy of herbal medicines and improve patient outcomes. As the field continues to evolve, the integration of AI with traditional medicinal practices will likely lead to more effective and individualized therapeutic strategies.
The application of AI in the prediction of diseases and optimization of treatment strategies using medicinal plants represents a transformative approach in the field of healthcare. By leveraging advanced computational techniques, researchers can enhance the efficacy of traditional herbal remedies, tailor treatments to individual patients, and improve overall health outcomes (Figure 7).
3.6.1. Methodologies for disease prediction
3.6.1.1. Data collection and integration. The first step in AI-driven disease prediction involves the collection of diverse datasets, including clinical data, genomic information, and phytochemical profiles of medicinal plants. For instance, the Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) provides a comprehensive resource with over 29,000 ingredients from approximately 500 herbal medicines, which can be correlated with various diseases (Allen, 2024). Similarly, the Indian Medicinal Plants, Phytochemistry, and Therapeutics (IMPPAT) database offers insights into the interactions between phytochemicals and human target proteins, facilitating the identification of potential therapeutic agents (Contreras and Vehı́, 2018).
3.6.1.2. Machine learning algorithms. ML algorithms are employed to analyze the collected data and identify patterns associated with specific diseases. For example, Hosny et al. (2018) demonstrated the potential of DL algorithms in predicting lung cancer outcomes by analyzing radiomic features from medical images (Deng et al., 2022). In the context of medicinal plants, ML can be used to correlate the presence of specific phytochemicals with disease outcomes, enabling the identification of plants that may be effective in treating particular conditions.
3.6.1.3. Predictive modeling. AI-driven predictive models can be developed to forecast disease progression and treatment responses. These models utilize historical data to generate insights into how patients may respond to various treatments based on their unique genetic and biochemical profiles. For instance, the use of AI in diabetes management has shown promise in personalizing treatment plans by analyzing patient data and predicting glycemic responses (Hosny et al., 2018). Such methodologies can be adapted to herbal medicine, allowing for the customization of treatment regimens based on individual patient characteristics.
3.6.2. Treatment optimization
3.6.2.1. Phytochemical profiling. The optimization of treatment strategies using medicinal plants involves detailed phytochemical profiling to identify bioactive compounds responsible for therapeutic effects. Advanced techniques such as GC-MS and HPLC are commonly used to analyze the chemical constituents of medicinal plants. By integrating these profiles with AI algorithms, researchers can predict the efficacy of specific compounds against various diseases (Prasher et al., 2023).
3.6.2.2. AI-enhanced decision support systems. AI can facilitate the development of decision support systems that assist healthcare providers in selecting the most appropriate herbal treatments for patients. For example, AI algorithms can analyze patient data, including genetic information and previous treatment responses, to recommend personalized herbal remedies. This approach aligns with the principles of precision medicine, which aims to tailor treatments to individual patient needs (Mohanraj et al., 2018).
3.6.2.3. Clinical trials and validation. The effectiveness of AI-driven treatment optimization must be validated through clinical trials. AI can streamline the design and analysis of these trials by identifying suitable patient populations and predicting outcomes based on historical data. For instance, the role of AI in managing glioma patients by analyzing extensive clinical and genetic databases to inform treatment planning was highlighted by Sotoudeh et al. (2019) and Abbas (2024). Such methodologies can be applied to herbal medicine, ensuring that the efficacy of plant-based treatments is rigorously evaluated.
Despite the promising advancements in AI-driven disease prediction and treatment optimization, several challenges remain. The integration of diverse datasets can be hindered by issues related to data standardization and quality (Bi, 2023). Additionally, the complexity of plant-based treatments, which often involve multiple active compounds, complicates the predictive modeling process.
Future research should focus on enhancing the robustness of AI algorithms and improving the integration of multiomics data (genomics, transcriptomics, and metabolomics) to provide a more comprehensive understanding of the interactions between medicinal plants and human health (Udegbe, 2024). Furthermore, the development of user-friendly AI tools for healthcare practitioners can facilitate the adoption of these technologies in clinical settings.
The methodologies for disease prediction and treatment optimization using medicinal plants and AI represent a significant advancement in healthcare. By integrating data from various sources and employing ML algorithms, researchers can enhance the efficacy of traditional herbal remedies and tailor treatments to individual patients. As AI technology continues to evolve, its application in the field of medicinal plants holds great promise for improving health outcomes and advancing the practice of precision medicine.
The integration of AI into the methodologies for disease prediction and treatment optimization using medicinal plants represents a significant advancement in healthcare. By leveraging AI technologies, researchers and healthcare practitioners can enhance the efficacy of traditional herbal remedies, tailor treatments to individual patients, and improve overall health outcomes.
3..7. Ethnobotanical data analysis of medicinal plants with AI
The analysis of ethnobotanical data using AI represents a significant advancement in understanding the medicinal properties of plants and their applications in traditional medicine. Ethnobotany, the study of the relationship between people and plants, provides a rich source of knowledge that can be systematically analyzed to uncover patterns and insights related to the use of medicinal plants. By leveraging AI technologies, researchers can enhance the extraction of valuable information from ethnobotanical datasets, leading to improved conservation strategies and the development of new therapeutic agents. AI techniques, particularly ML, have proven effective in processing large volumes of ethnobotanical data. For example, ML algorithms can identify patterns in the usage of medicinal plants across different cultures and geographical regions. This capability allows researchers to analyze the frequency of plant use, the diversity of applications, and the demographic factors influencing these practices (Alibek et al., 2021). In a study conducted in Ethiopia, both qualitative and quantitative analytical tools were employed to summarize descriptive ethnobotanical data, demonstrating the effectiveness of combining traditional ethnobotanical methods with modern data analysis techniques (Prasher et al., 2023). Such approaches can reveal important correlations between local ecological knowledge and the medicinal properties attributed to specific plants. Furthermore, AI can facilitate the integration of historical ethnobotanical data with contemporary research. For instance, historical records from regions like Eastern Europe have been systematically analyzed to understand how local ecological knowledge has evolved over time (Kefalew et al., 2015). By applying AI to these datasets, researchers can uncover trends in plant usage and the shifting perceptions of medicinal plants within communities. This historical perspective enriches current ethnobotanical studies by providing context and depth to the understanding of plant-based therapies. The application of AI in ethnobotanical data analysis also extends to the evaluation of the efficacy and safety of medicinal plants. By analyzing large datasets from ethnobotanical surveys, researchers can identify plants that are frequently reported for specific ailments, such as gastrointestinal complaints (Liswandari, 2024). This data-driven approach can guide further pharmacological studies and clinical trials, ultimately leading to the validation of traditional uses and the discovery of new therapeutic compounds. For example, a survey in Morocco quantified the ethnobotanical knowledge of plants used by traditional healers to combat COVID-19, employing quantitative analysis to assess the plant use value (Prakofjewa et al., 2022). Such studies highlight the potential of AI to bridge the gap between traditional knowledge and modern scientific validation. Moreover, AI can enhance the participatory aspect of ethnobotanical research by facilitating community engagement in data collection and analysis. By using mobile applications and online platforms, researchers can gather ethnobotanical data directly from local populations, ensuring that the knowledge of traditional healers and community members is accurately represented (Tariq et al., 2015). AI algorithms can then analyze this data to identify key plants and their uses, fostering a collaborative approach to conservation and sustainable use of medicinal plants. In conclusion, the integration of AI in the analysis of ethnobotanical data offers a powerful tool for understanding the complex relationships between humans and medicinal plants. By leveraging AI technologies, researchers can uncover valuable insights that inform conservation strategies, validate traditional medicinal practices, and promote the sustainable use of plant resources. As the field of ethnobotany continues to evolve, the application of AI will undoubtedly play a crucial role in enhancing our understanding of the medicinal properties of plants and their significance in traditional medicine.
The integration of AI into ethnobotanical data analysis of medicinal plants has emerged as a transformative approach, enhancing our understanding of traditional knowledge and its applications in modern medicine (Figure 8).
3.7.1. Methodologies for ethnobotanical data analysis
3.7.1.1. Data collection and documentation. The foundation of ethnobotanical research lies in the systematic collection of data regarding the uses of plants by local communities. This involves ethnographic methods such as interviews, surveys, and participatory observations to gather information on plant species, their medicinal uses, and the cultural significance attributed to them. For instance, Luo et al. (2019) documented the diversity and use of medicinal plants in traditional diets, highlighting the importance of local ethnobotanical knowledge in culinary practices. Such qualitative data serves as a critical resource for understanding the relationship between communities and their medicinal flora.
3.7.1.2. Database creation and management. Once data is collected, it is essential to organize it into accessible databases. These databases can include information on plant taxonomy, traditional uses, preparation methods, and associated ailments. The establishment of comprehensive databases facilitates the integration of ethnobotanical knowledge with modern scientific research. For example, the creation of digital ethnobotanical gardens can promote the conservation of traditional knowledge while providing educational resources for future generations (Xie et al., 2023).
3.7.1.3. Machine learning and predictive modeling. AI methodologies, particularly ML algorithms, play a crucial role in analyzing ethnobotanical data. Böhlen and Sujarwo (2020) emphasized the potential of ML in documenting ethnobotanical knowledge, enabling researchers to capture and analyze data that might otherwise remain unrepresented. These models can identify patterns and correlations within large datasets, enabling researchers to predict the bioactivity of plant species based on their ethnobotanical uses. For instance, Greene et al. (2023) discussed the use of photographic voucher specimens combined with ML to enhance plant identification efficiency, thereby streamlining data collection in ethnobotanical research.
3.7.1.4. Quantitative ethnobotanical analysis. Quantitative methods are essential for assessing the significance of specific plants within a community’s medicinal repertoire. Techniques such as the Informant Consensus Factor (ICF), Fidelity Level Index (FLI), and direct matrix ranking can be employed to evaluate the effectiveness and popularity of medicinal plants among local practitioners. For example, Xie et al. (2023) analyzed wild medicinal plant resources in multiethnic areas, revealing the most frequently used plants for various ailments. This quantitative approach provides a robust framework for understanding the cultural importance of medicinal plants and their potential for further research.
3.7.1.5. Geospatial analysis. The application of geospatial technologies, such as Geographic Information Systems (GIS), allows researchers to visualize the distribution of medicinal plants and their uses across different regions. This spatial analysis can reveal patterns related to biodiversity, habitat preferences, and the impact of environmental factors on plant availability. By integrating geospatial data with ethnobotanical knowledge, researchers can identify areas rich in medicinal plant diversity and prioritize them for conservation efforts.
3.7.2. Applications and benefits of ethnobotanical data analysis
3.7.2.1. Drug discovery and development. The integration of AI in ethnobotanical studies can significantly contribute to drug discovery. By analyzing traditional uses of plants and correlating them with chemical compounds, researchers can identify potential lead compounds for pharmaceutical development. This approach not only accelerates the discovery of new medicines but also promotes the sustainable use of biodiversity (Liu, 2024).
3.7.2.2. Conservation of traditional knowledge. AI-driven ethnobotanical data analysis plays a vital role in preserving traditional knowledge systems. By documenting and analyzing local practices, researchers can ensure that valuable ethnobotanical knowledge is not lost to modernization and urbanization. This preservation is crucial for maintaining cultural heritage and promoting biodiversity conservation (Shah et al., 2023).
3.7.2.3. Education and community engagement. The use of AI in ethnobotanical research can enhance educational initiatives aimed at revitalizing traditional knowledge among younger generations. Programs that incorporate local plant knowledge into school curricula can foster appreciation for biodiversity and encourage sustainable practices (Dunjic, 2024). Engaging communities in ethnobotanical research also empowers them to take an active role in conserving their natural resources.
Despite the advancements in AI-driven ethnobotanical data analysis, challenges remain. Issues related to data standardization, quality, and accessibility can hinder the integration of diverse datasets. Additionally, the complexity of traditional knowledge systems requires careful consideration to avoid oversimplification or misrepresentation of cultural practices.
Future research should focus on developing user-friendly AI tools that facilitate data collection and analysis for ethnobotanists and local communities. Collaborative efforts between scientists, local practitioners, and policymakers can enhance the effectiveness of ethnobotanical studies and promote the sustainable use of medicinal plants.
The methodologies for ethnobotanical data analysis using AI offer powerful tools for understanding the intricate relationships between people and plants. By integrating traditional knowledge with modern scientific approaches, researchers can uncover the potential of medicinal plants for drug discovery, promote conservation efforts, and revitalize cultural heritage. As AI technology continues to evolve, its application in ethnobotany holds great promise for advancing our understanding of medicinal plants and their role in human health.
3.8. Clinical trials and predictive modeling of medicinal plants with AI
The integration of AI into clinical trials and predictive modeling of medicinal plants represents a significant advancement in the field of herbal medicine. This approach enhances the efficiency of clinical research and improves the accuracy of predictions regarding the efficacy and safety of herbal treatments. By leveraging AI technologies, researchers can analyze complex datasets, optimize trial designs, and ultimately facilitate the development of effective herbal therapies. AI has the potential to transform clinical trials involving medicinal plants by streamlining the recruitment process, monitoring patient outcomes, and analyzing trial data. For instance, AI algorithms can be employed to identify suitable candidates for clinical trials based on specific inclusion and exclusion criteria, thereby accelerating patient recruitment (Imanaliyeva et al., 2024). Moreover, AI can assist in real-time monitoring of patient responses to herbal treatments, allowing for adaptive trial designs that can modify treatment protocols based on interim results. This flexibility is particularly beneficial in trials involving herbal medicines, where patient responses may vary significantly due to individual differences in metabolism and genetic predispositions. Predictive modeling is another area where AI can significantly impact the clinical application of medicinal plants. By analyzing historical clinical trial data, AI models can identify patterns that predict treatment outcomes, helping researchers to refine their hypotheses and improve the design of future studies. For example, ML techniques can be utilized to predict the likelihood of adverse reactions to specific herbal compounds, enabling researchers to assess the risk-to-benefit ratio more effectively (Mobasheri, 2012). This predictive capability is crucial for ensuring patient safety and optimizing treatment protocols. Furthermore, systematic reviews and meta-analyses of clinical trials involving herbal medicines can benefit from AI-driven data analysis. For instance, studies have demonstrated the efficacy of various herbal treatments for conditions such as xerostomia in cancer patients and chemotherapy-induced peripheral neuropathy (Noh et al., 2018; Rajkovic et al., 2023). By employing AI to aggregate and analyze data from multiple trials, researchers can derive more robust conclusions regarding the effectiveness of these treatments, ultimately guiding clinical practice and informing healthcare providers about the best therapeutic options available. AI can also facilitate the exploration of the mechanisms of action of medicinal plants. By integrating ethnobotanical data with clinical trial results, researchers can identify specific phytochemicals responsible for therapeutic effects and their interactions with biological targets (Willcox et al., 2011). This holistic understanding of how herbal medicines work can lead to the development of more targeted and effective treatment strategies, particularly in complex diseases such as cancer, where combination therapies involving both herbal and conventional medicines are gaining traction (Rajkovic et al., 2023). Moreover, the use of AI in clinical trials can enhance the understanding of patient populations and their responses to herbal treatments. By analyzing demographic and clinical data, AI can identify subgroups of patients who may benefit most from specific herbal therapies, thus promoting personalized medicine approaches (Zahra et al., 2023). This is particularly relevant in the context of traditional herbal medicine, where cultural and regional factors can influence treatment efficacy and patient acceptance. In conclusion, the application of AI in clinical trials and predictive modeling of medicinal plants holds great promise for advancing the field of herbal medicine. By enhancing the efficiency of clinical research, improving patient safety, and facilitating the development of targeted therapies, AI can play a crucial role in the integration of herbal medicine into mainstream healthcare. As research continues to evolve, the collaboration between AI technologies and traditional herbal practices will likely lead to more effective and personalized treatment options for patients.
The application of AI in clinical trials and predictive modeling of medicinal plants is revolutionizing the field of pharmacology and personalized medicine. By harnessing the power of AI, researchers can enhance the efficiency of clinical trials, improve the accuracy of predictive models, and ultimately optimize the therapeutic potential of medicinal plants (Figure 9).
3.8.1 Methodologies for clinical trials involving medicinal plants
3.8.1.1. Study design and protocol development. The design of clinical trials involving medicinal plants must adhere to established guidelines to ensure rigorous and reproducible results. The Consolidated Standards of Reporting Trials (CONSORT)-AI extension provides a framework for reporting trials that involve AI interventions, emphasizing the importance of transparency in methodology (Liu et al., 2020). This includes detailing the AI algorithms used, the data inputs, and how the intervention is integrated into the trial. Additionally, the SPIRIT-AI extension outlines the necessary components for trial protocols, ensuring that AI interventions are adequately described and validated (Rivera et al., 2020).
3.8.1.2. Patient recruitment and data collection. AI can streamline the recruitment process by analyzing patient data to identify suitable candidates for clinical trials. By examining electronic health records and other medical data, AI algorithms can match patients with specific eligibility criteria, thereby enhancing recruitment efficiency (Unogwu, 2023). Furthermore, AI can facilitate real-time data collection during trials, utilizing wearable devices and mobile applications to monitor patient responses and adherence to treatment protocols.
3.8.1.3. Predictive modeling. AI-driven predictive modeling plays a crucial role in clinical trials by forecasting patient outcomes based on historical data. ML algorithms can analyze vast datasets to identify patterns and correlations that inform treatment efficacy. For instance, predictive models can estimate the likelihood of adverse reactions to medicinal plants, allowing researchers to adjust treatment protocols accordingly (Askin et al., 2023). This capability is particularly valuable in the context of personalized medicine, where individual patient characteristics significantly influence treatment responses.
3.8.1.4. Data analysis and interpretation. The analysis of clinical trial data can be significantly enhanced through AI methodologies. Advanced statistical techniques such as DL and NLP can be employed to analyze complex datasets, including genomic, proteomic, and clinical data (Cunningham et al., 2024). For example, NLP can be used to extract relevant information from clinical notes, facilitating a more comprehensive understanding of patient responses to medicinal plant treatments. This integration of AI in data analysis not only improves the accuracy of findings but also accelerates the timeline for bringing new treatments to market.
3.8.2 Applications of predictive modeling in medicinal plants
3.8.2.1. Bioactivity prediction. Predictive modeling can be utilized to assess the bioactivity of various phytochemicals found in medicinal plants. By correlating chemical structures with biological activity, AI algorithms can predict which compounds are likely to exhibit therapeutic effects. This approach can significantly expedite the drug discovery process by narrowing down the list of candidate compounds for further investigation (Zhi et al., 2021).
3.8.2.2. Personalized treatment plans. AI-driven predictive models can inform personalized treatment strategies by analyzing patient-specific data, including genetic profiles and previous treatment responses. This allows healthcare providers to tailor medicinal plant therapies to individual patients, optimizing efficacy and minimizing adverse effects (Aitbekov et al., 2024). For instance, predictive modeling can help identify which patients are most likely to benefit from a specific herbal remedy based on their unique biological characteristics.
3.8.2.3. Risk assessment and management. Predictive modeling can also be employed to assess the risks associated with the use of medicinal plants. By analyzing historical data on adverse events and patient outcomes, AI algorithms can identify potential safety concerns and inform risk management strategies (Chen et al., 2025). This is particularly important in the context of herbal medicine, where the safety profiles of many plants are not well established.
Despite the promising advancements in AI-driven clinical trials and predictive modeling, several challenges remain. Issues related to data quality, standardization, and accessibility can hinder the integration of diverse datasets. Additionally, the complexity of AI algorithms may pose challenges in terms of interpretability and transparency, which are critical for regulatory approval and clinical acceptance (Plana et al., 2022).
Future research should focus on enhancing the robustness of AI algorithms and improving the integration of multiomics data (genomics, transcriptomics, and metabolomics) to provide a more comprehensive understanding of the interactions between medicinal plants and human health. Furthermore, the development of user-friendly AI tools for healthcare practitioners can facilitate the adoption of these technologies in clinical settings.
The methodologies for clinical trials and predictive modeling involving medicinal plants when combined with AI offer powerful tools for optimizing therapeutic outcomes. By integrating AI into the clinical trial process, researchers can enhance patient recruitment, improve data analysis, and develop personalized treatment strategies. As AI technology continues to evolve, its application in the field of medicinal plants holds great promise for advancing healthcare and improving patient outcomes.
3.9. Personalized medicine of medicinal plants with AI
The advent of AI in personalized medicine, particularly concerning medicinal plants, heralds a new era in healthcare that emphasizes tailored treatment strategies based on individual patient characteristics. Personalized medicine aims to optimize therapeutic efficacy while minimizing adverse effects, and the integration of AI technologies into this field enhances the ability to analyze complex datasets, predict treatment outcomes, and identify the most suitable herbal interventions for patients. Medicinal plants are a rich source of bioactive compounds that have been utilized for centuries in traditional medicine. The pharmacological properties of these plants can vary significantly based on genetic, environmental, and lifestyle factors of the individuals using them. AI can facilitate the identification of these variables through predictive modeling, which analyzes patient data to determine the most effective herbal treatments for specific conditions. For instance, studies have shown that certain medicinal plants, such as Withania somnifera (Ashwagandha), exhibit varying levels of efficacy in treating conditions like stress and anxiety, depending on individual patient profiles (Ahmed and El-Darier, 2024). By employing ML algorithms, researchers can analyze historical data to predict which patients are likely to benefit most from specific herbal therapies. Moreover, AI can enhance the understanding of the mechanisms through which medicinal plants exert their effects. By integrating data from ethnobotanical studies, clinical trials, and phytochemical analyses, AI can help elucidate the pathways through which herbal compounds interact with biological systems. This holistic approach is particularly valuable in the context of chronic diseases such as diabetes, where personalized treatment plans can be developed based on individual responses to herbal remedies (Ardakani, 2022; Sheikhrabori et al., 2017). For example, the use of cinnamon and other medicinal plants has been shown to significantly reduce blood sugar levels in diabetic patients, but the effectiveness can vary based on genetic predispositions and lifestyle factors (Sheikhrabori et al., 2022). AI-driven predictive modeling can also play a crucial role in identifying potential adverse effects associated with herbal treatments. By analyzing large datasets from clinical trials and patient reports, AI can help identify patterns that may indicate risks or contraindications for specific patient populations. This capability is essential for ensuring patient safety and optimizing treatment protocols, particularly in populations with diverse genetic backgrounds and health conditions (Bukhsh et al., 2018; Raja, 2015). For instance, understanding the barriers to effective diabetes management among patients using complementary and alternative medicine can inform healthcare providers about the best practices for integrating herbal treatments into conventional care (Bukhsh et al., 2020). Furthermore, the application of AI in personalized medicine extends to the development of mobile health applications that can monitor patient responses to herbal treatments in real time. These applications can collect data on patient symptoms, medication adherence, and lifestyle factors, allowing for continuous feedback and adjustments to treatment plans. This dynamic approach not only enhances patient engagement but also provides healthcare providers with valuable insights into the effectiveness of herbal interventions (Mohammed and Amsalu, 2023). The potential of AI in personalized medicine is further exemplified by its ability to analyze the cultural and contextual factors influencing the use of medicinal plants. Understanding the sociocultural determinants of health can inform the development of personalized treatment plans that are culturally sensitive and more likely to be accepted by patients (Al-Zeidaneen, 2017; Husain, 2023). For example, studies have shown that the habitual use of medicinal plants among elderly populations is influenced by factors such as physical activity and gender, highlighting the importance of tailoring interventions to meet the specific needs of diverse patient groups (Al-Zeidaneen et al., 2019). In conclusion, the integration of AI into personalized medicine, particularly in the context of medicinal plants, offers a transformative approach to healthcare. By leveraging AI technologies to analyze complex datasets, predict treatment outcomes, and personalize herbal interventions, healthcare providers can enhance the efficacy and safety of treatments. As research continues to evolve, the collaboration between AI and traditional herbal practices will likely lead to more effective and individualized therapeutic strategies, ultimately improving patient outcomes and quality of life.
The integration of AI in personalized medicine, particularly concerning medicinal plants, is reshaping the landscape of healthcare. By leveraging AI technologies, researchers and healthcare providers can tailor treatments based on individual patient profiles, enhancing therapeutic efficacy and minimizing adverse effects (Figure 10).
3.9.1. Methodologies for personalized medicine with medicinal plants
3.9.1.1. Data collection and integration. The first step in implementing personalized medicine is the comprehensive collection of data, which includes genomic, proteomic, and metabolomic information from patients, as well as ethnobotanical data regarding medicinal plants. Databases such as the TCMSP provide extensive information on plant compounds and their therapeutic effects (Ravipati et al., 2012). Integrating these datasets allows researchers to correlate specific genetic profiles with the efficacy of various medicinal plants.
3.9.1.2. Machine learning and predictive modeling. AI-driven ML algorithms are pivotal in analyzing the collected data to identify patterns and predict treatment outcomes. For example, predictive models can be developed to assess how different patients may respond to specific herbal remedies based on their genetic makeup and health conditions. This approach has been successfully applied in studies examining the immunomodulatory effects of Ocimum sanctum (Tulsi), where ML was used to analyze cytokine levels and immune responses (Mondal et al., 2011). Such predictive modeling enables healthcare providers to customize treatment plans that align with individual patient needs.
3.9.1.3. Phytochemical profiling. Understanding the bioactive compounds present in medicinal plants is crucial for personalized medicine. Advanced techniques such as HPLC and GC-MS are employed to profile the phytochemicals in these plants. By integrating phytochemical data with patient-specific information, AI can help identify which compounds are most likely to be effective for particular health conditions. For instance, the antioxidant and anti-inflammatory properties of various Chinese medicinal plants have been linked to their phenolic content, which can be analyzed using AI algorithms to predict therapeutic outcomes (Ravipati et al., 2012).
3.9.1.4. Clinical trials and real-world evidence. AI can enhance the design and execution of clinical trials involving medicinal plants. By utilizing AI algorithms to analyze historical trial data, researchers can identify optimal dosing regimens and patient populations for new herbal treatments. Additionally, AI can facilitate the collection of real-world evidence from patients using medicinal plants, providing insights into long-term efficacy and safety. This data can be invaluable in refining treatment protocols and ensuring that they are tailored to the needs of diverse patient populations (Ahmed, 2021).
3.9.2. Applications of AI in personalized medicine with medicinal plants
3.9.2.1. Customized treatment plans. AI enables the development of personalized treatment plans that consider individual patient characteristics, including genetic predispositions, lifestyle factors, and existing health conditions. For example, patients with specific genetic markers may respond better to certain phytochemicals found in medicinal plants, allowing healthcare providers to tailor herbal remedies accordingly. This personalized approach not only enhances treatment efficacy but also reduces the risk of adverse reactions (Khossravi, 2024).
3.9.2.2. Drug discovery and development. The integration of AI in drug discovery processes can expedite the identification of new therapeutic agents derived from medicinal plants. By analyzing vast datasets of plant compounds and their biological activities, AI can predict which compounds are most likely to yield effective treatments for specific diseases. This capability is particularly valuable in the context of increasing interest in natural products as alternatives to synthetic pharmaceuticals (Paudel et al., 2018).
3.9.2.3. Monitoring and feedback systems. AI can facilitate the development of monitoring systems that track patient responses to herbal treatments in real time. By collecting data on patient health metrics and treatment adherence, AI algorithms can provide feedback to healthcare providers, enabling timely adjustments to treatment plans. This dynamic approach ensures that patients receive the most effective therapies based on their ongoing health status (Gandhi, 2024).
Despite the promising advancements in personalized medicine through AI and medicinal plants, several challenges remain. Data quality and standardization are critical issues that can hinder the integration of diverse datasets. Additionally, the complexity of AI algorithms may pose challenges in terms of interpretability and transparency, which are essential for regulatory approval and clinical acceptance (Rane et al., 2024).
The methodologies for personalized medicine involving medicinal plants when combined with AI offer powerful tools for optimizing therapeutic outcomes. By integrating AI into the clinical trial process and predictive modeling, researchers can enhance patient recruitment, improve data analysis, and develop tailored treatment strategies. As AI technology continues to evolve, its application in the field of medicinal plants holds great promise for advancing healthcare and improving patient outcomes.
CONCLUSION
An important advancement in speeding up the identification, categorization, and therapeutic uses of plant-based chemicals is the incorporation of AI into medicinal plant research. Researchers can expedite the process of finding medicinal plants, evaluating their bioactive compounds, and forecasting their therapeutic potential by employing AI-driven approaches, including ML, DL, and NLP. The precision of plant identification, biodiversity assessment, and disease prediction and treatment strategies based on medicinal plant qualities have all benefited greatly from this technical breakthrough. AI also makes it easier to analyze ethnobotanical data, which supports the sustainable use of plant species and allows for the preservation of traditional knowledge.
AI-powered technologies for drug discovery, virtual screening, and phytochemical analysis are transforming the creation of new treatments by providing more effective and economical approaches than conventional research methods. AI-powered personalized medicine is also making progress in tailoring treatment regimens to each patient’s needs, improving therapeutic results. Even with all the benefits, there are still issues, especially with data integration, model accuracy, and the requirement for more thorough clinical studies. AI has enormous potential to overcome these obstacles, advance the study of medicinal plants, and support ecological conservation and global health initiatives as it develops further. In summary, the combination of AI and medicinal plant research not only speeds up the identification of promising therapeutic compounds but also offers a fresh approach to long-term healthcare solutions. Medicinal plants will continue to be a vital resource for upcoming medical advancements as AI technologies advance and present previously unheard-of chances for invention.