INTRODUCTION
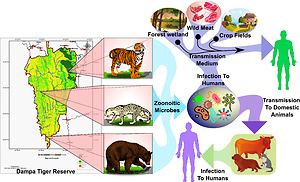
Natural systems are interacting, dynamics assemblages of varied species and ecological processes within. Microbes are an integral part of these systems, playing crucial roles in the biology of other organisms and contributing to the regulation of the overall systems (Habtamu et al., 2017; Hauffe & Barelli, 2019; Ruth et al., 2008; Stuart et al., 2013). Gut microbiota is personified with different functions of host-microbe interactions such as ecology, diet or food selection, behaviour, host immune system and the overall health of the host (Bahrndorff, Alemu, Alemneh, & Nielsen, 2016; Leeming et al., 2021). However, as animals and humans have come to live in close proximity and there are cases of frequent encroachments into one other territory; microbial parasites are now viewed as potential zoonotic agents for human wellbeing (Gibb et al., 2020; Jones et al., 2008; Keatts et al., 2021). The majority of emerging infectious diseases (over 60%) are considered zoonotic. Zoonoses contribute an estimated 75% of new or re-emerging infectious diseases in humans (Recht, Schuenemann, & Sánchez-Villagra, 2020). The most devastating pandemics in human history, the Black Death (in the 1300s), Spanish influenza (1918), and Human Immunodeficiency Virus/ Acquired Immunodeficiency Syndrome (HIV/AIDS) (Early 2000s), Middle East respiratory syndrome (MERS) (2012), Covid-19, all resulted from an initial zoonotic spillover from wildlife (Jones et al., 2008; Keatts et al., 2021; Keesing & Ostfeld, 2021). It has been estimated that about 43% of the zoonotic viral, protozoan, bacterial, and fungal human infections naturally originated from carnivore hosts (Cleaveland, Laurenson, & Taylor, 1411; Johnson et al., 2015; Woolhouse & Sequeria, 2005). Carnivores can act as definitive hosts and reservoirs for many intestinal parasites; some of them are responsible for diseases such as paragonimiasis, sarcocystosis, toxoplasmosis and cysticercosis (Beltrame, Bellusci, Fernández, & Sardella, 2018). A diverse suite of parasites release propagates in the form of eggs, larvae, and oocysts in fecal matters that can be easily transmitted into humans through various modes like soil, water, vegetables, and domesticated animals (Plowright et al., 2021). Escalating anthropogenic activities are increasing contact rates between humans, domestic animals, and wildlife. These rises have been linked to changing human ecology, growing human population, and its demand for bushmeat, wild animals as pets, agricultural land, natural resources, shrinking of wildlife habitats etc. (Gibb et al., 2020; Herrera & Nunn, 2019; Jones et al., 2008; Plowright et al., 2021).
With the development of ‘One health movement’ more focus has been laid on the recognition of wildlife as a major source of zoonotic infections, thereby encouraging intensive research in the detection of wildlife pathogens (Nath et al., 2021; Wicker, Canfield, & Higgins, 2017). Given the potential for transmission of diseases between wildlife, humans and livestock, an attempt was made to study the prevalence of parasites in wild carnivores of Dampa Tiger Reserve in Mizoram, India and its potential implications on human health.
MATERIALS AND METHODS
Study area and sample collection
The study was carried out in Dampa Tiger Reserve (DTR) (Longitude 92013'12" to 92027'24" E & latitude 230 32' 42" to 230 41' 36" N), the largest protected area of the state of Mizoram, India. The reserve stretches over an area of 948 sq. km (500 sq. km as core & 448 sq. km as buffer area) (Figure 1).
The reserve harbours a diverse range of carnivores namely Tiger (Panthera tigris tigris), Clouded leopard (Neofelis nebulosa), Large Indian civet (Viverra zibetha), Small Indian civet (Virerricula indica), Golden jackal (Canis aureus), Dhole (Cuon alpinus), Marbled cat (Pardofelis marmorata), Leopard cat (Prionailurus bengalensis) etc. (Singh & Macdonald, 2017). The reserve is surrounded by 15 fringe villages with a population of over 22,500 that rely on shifting cultivation and other forest products for their livelihood (Gouda, Decemson, Parida, & Solanki, 2020). With agricultural crop fields, adjacent to the tiger reserve, many of these carnivore species often move across for better feeding opportunities. Eighty-four fresh fecal samples of different wild carnivores (Clouded leopard, leopard cat, civets etc.) were collected from DTR and its adjoining buffer areas while working on predefined forest trails over the period of six months. About 20g of freshly or old voided fecal sample of each animal were collected in an individually labelled polythene bags, and the samples were properly sealed for future studies. Samples were stored in 10% formalin at sampling site. The samples were shaken vigorously to maximize contact between sample and storage solution.
Coprological analysis
The collected fecal samples were examined for the presence of enteric parasitic eggs/ oocysts by direct smear examination, floatation techniques and fecal sedimentation process following Gillespie (2006). All observations were made using a compound microscope at 10X and 40X objectives. Identification and confirmation of the parasites were made by an experienced diagnostic parasitologist from Veterinary College and Animal Husbandry, Aizawl, Mizoram.
RESULTS
A total of eighty-four fecal samples were collected during the survey in DTR and its surrounding areas. The sample comprises of the scats of Canidae (19 nos.), Erentizontidae (2 nos.), Felidae (42 nos.), Herpestidae (8 nos.), Sciucridae (2 nos.), Soricidae (1 nos.) and Viverridae (8 nos.). The overall prevalence of intestinal parasitic infection in the study was recorded to be 90.47%, as 76 samples tested positive with ova of parasites. Eight samples tested negative for any forms of pathogens. About 61.90% of the total samples tested positive for helminths, while, 38.09% of the samples tested positive for protozoans.
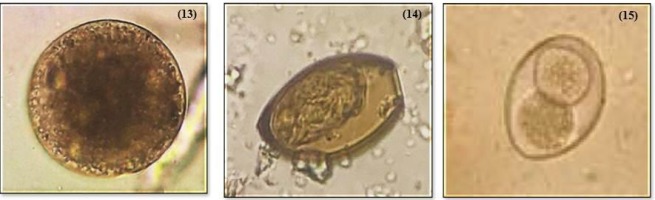
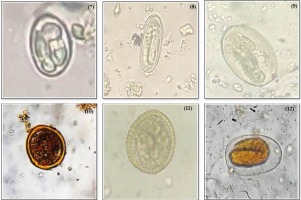
A total of 15 parasites were identified from the examined fecal samples that include Diphyllobothrium spp., Capllaria spp., Taenia spp., Trichiuris trichiura, Acanthosephala spp., Physaloptera spp., Eimeria spp., Spirometra spp., Paragonimus spp., Ascaris lumbricoides, Toxocara spp. Strongyle spp., Balantidium coli, Opisthorchis spp., and Isospora spp. (Figure 2-4).
Figure 2
(1) Diphyllobothrium spp. (2) Capllaria spp. (3) Taenia spp. (4) Trichuris spp. (5) Acanthosephala spp. (6) Physaloptera spp.
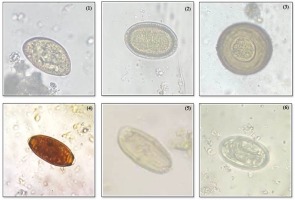
Figure 3
(7) Eimeria spp. (8) Spirometra spp. (9) Paragonimus spp. (10) Ascaris lumbricoides (11) Toxocara spp. (12) Strongyle spp.
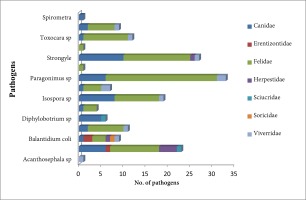
The parasite Paragonimus spp. was recoded to be present in highest number i.e. 33 nos. of scat samples followed by Strongyle spp. (27 nos.), Ascaris lumbricoides (23 nos.), and Isospora spp. (19 nos.). The pathogen B. coli, although observed in lesser number, was found to be the most diverse parasite occurring in six of the seven groups of carnivores fecal matters followed by A. lumbricoides (5/7). Pathogen like Spirometra sp, Taenia spp., Acanthosephala spp., and Physaloptera spp. were observed only once in the tested samples. In the family Felidae, parasitic prevalence was recorded in the largest number followed by Canidae and Viverridae with 97 nos., 45 nos. and 11 nos. respectively. The species Paragonimus spp. was found to infect mostly the members of the family Felidae, while the species Strongyle spp. mainly infects the Canidae members (Table 1, Figure 5).
Table 1
Overall prevalence of parasite in the testedfecal samples of carnivore species from Dampa Tiger Reserve
Figure 5
Diversity and distribution of gastrointestinal parasites recorded from scat samples of carnivores collected from Dampa Tiger Reserve and its surrounding areas
Prevalence of parasite among different families of host species was checked again by Kruskal wallis test to see if there is any form of variation, to which highly significant variation was observed i.e., x2= 46.94, df= 6, and P < 0.001. Mann-Whitney test was then performed to see variation on the infection rate of the parasite in the pair of each family. The Pairwise family comparison by Mann-Whiney and Wilcoxon W showed significant variations between the families of Canidae vs Herpestidae, Canidae vs Sciuridae, Canidae vs Soricidae, Erentizontidae vs Felidae, Felidae vs Sciuridae, Canidae vrs. Erentizontidae with P value < 0.001.
Potential health implications of the identified parasites/ zoonoses from the examined scat samples of carnivores on human health were studied through available literatures. The pathogens mostly use the carnivores as intermediate hosts and reaches humans through different modes like faecal-oral route, uncooked meat, fishes, contaminated water etc., causing moderate to severe health complications (Table 2).
Some of the important pathogenic strains include the Spirometra spp., Paragonimus spp., Capillaria spp., Toxocara spp., A. lumbricoides and Opisthorchis spp. causing inflammatory in brain, symptoms similar to tuberculosis, bronchitis, covert toxocariasis, acute lung inflammation etc. The pathogens, namely Acanthocephala spp. Eimeria and Isospora spp., although have rare chances of inflecting diseases in humans, can infect wildlife, along with economically significant species such as rabbits, cattle, sheep, pigs. These pathogens often proliferate in human-dominated landscapes, thereby increasing the likelihood of spillover.
DISCUSSION
Zoonotic studies were mostly focused on zoos considering the exposure risk to the general public, whereas studies on parasitic diversity among carnivores in the wild are limited (Dib et al., 2020; Figueiredo et al., 2021).
Table 2
Summary of zoonoses identified in the study and their implications in human health
Zoonoses | Host species | Diseases in humans and its severity | Mode of infection | References |
Diphyllobothrium spp. | Species like D.caninum, D. felis, D. latum and D. lineatus are found among felids like the Hyena, Leopard, Jackal, etc. from India | Causes Human diphyllobothriasis with relatively minor clinical consequences | It is the longest parasite that infects humans. They infect humans upon accidentally ingestion of plerocercoids of Diphyllobothrium spp. (Cestoda, Pseudophyllidea) while eaten raw, undercooked or sometimes smoked fish or other intermediate hosts. Most common species to infect humans is the D. latum. | (Acharjyo, 2004; Diemert, 2017; Luiz, Tavares, Luque, Teresa, & Bomfim, 2005) |
Spirometra spp. | Spirometra spp. are prevalent mainly in lion and civets | Usually results in subcutaneous inflammatory swelling, however if migrated to brain may result in devastating results | Humans become infected by consumption of raw or undercooked flesh of an intermediate host such as frog or snake, and small crustaceans | |
Paragonimus spp. | P. westermanii is the most commonly encountered species in Indian wild carnivores | Infections may results in symptoms similar to tuberculosis | Definitive hosts usually become infected by eating raw or undercooked tissues from crustaceans, such as snails, crabs and crayfish | |
Physaloptera spp. | Physaloptera are parasitic nematodes mostly detected in carnivores like leopards, and other feline species | P. praeputialis and P. rara re known to infect humans on rare occasions. It may cause mild symptoms. In children, it sometimes led to ulcer and inflammatory bowels. | Ingestion of intermediate or paratenic hosts, contaminated soil and water | |
Acanthocephala spp. | Prosthenorchis fraternal are reported from Jungle cat and other felids species | Although very rare, Acantocephalan do infects human with symptoms like abdominal pain, diarrhea and anorexia with weight loss | Consumption of infected fish, crustaceans, arthropods and other vertebrates | |
Strongyloidea spp. | S. akbari amd S. tumefacians are common in honey badger and leopard cat | In humans it cause different pictures of skin, enteric and pulmonary diseases | They are known to accessing to the definitive host through skin penetration or via ingestion. Humans may become infected; ingesting unwashed vegetables contaminated by animal faeces or contaminated water containing strongyloid larvae | |
Capillaria spp. | They are prevalent in jungle cat, crab eating mongoose and leopard cats | Capillaria are known to cause Hepatic capillariosis in humans. C. aerophilus and C. hepatica can result in bronchitis, coughing, mucoid sputum, fever, dyspnea etc. | Mode of infection are contaminated water, rodents and other domesticated animals | |
Toxocara spp. | T. cati, T. canis, T. mystax are common in felids of India. These species also act as indicator of overlapping between sylvatic and domestic animals species | Infections with Toxocara spp. Causes toxocariasis in humans leading to Visceral larva migrans (VLM), Ocular larva migrans (OLM) and Covert toxocariasis (CT). | Humans can be infected by ingestion of larvae in undercooked infected organ or muscle tissues (rare); infective eggs from contaminated soil (gardens, sandpits and playgrounds); from unwashed hands or raw vegetables | (Acharjyo, 2004; Beltrame et al., 2018; Figueiredo et al., 2021) |
Taenia spp. | T. hydatigena, T. pisiformis and T. taeniaformis an obligate parasite species of carnivores already reported from tiger, clouded leopard, jungle cat and jackals in India | Clinical consequences of Taeniasis are mild and are limited to minor abdominal symptoms, such as epigastric discomfort, nausea and vomiting | Human infection is through raw or undercooked pork liver | |
Eimeria and Isospora spp. | Eimeria spp. and Isospora spp. are ubiquitous coccidian present in common palm civet, deer etc. | Asymptomatic | Infection in humans is very rare, but causes great economic loss, reducing productivity and compromising animal welfare. | |
Trichinella spp. | T. spiralis is quite common in wild carnivores like civets and wild cats in India | Trichinellosis caused by Trichuris sp. modulates the gastrointestinal (GI) mucosa environment, causing it to be less hostile for worm development and survival | The infection is generated through the ingestion of an embryonated egg from soil sample | |
Ascaris lumbricoides | A. lumbricoides and A. suum are known to infect Canis familiaris, Leopardus pardalis, and Sus scrofa | A. lumbricoides are the causative agents of Ascariasis in humans. Infected person tends to experiences acute lung inflammation, difficulty in breathing and fever, nausea and diarrhea | Infection occurs through faecal-oral route, uncooked pork meat, unhygienic defecation practices etc. | (Dold & Holland, 2010; Otranto & Deplazes, 2019; Scott, 2008) |
Balantidiosis spp. | Balantidiosis spp. are reported from wild boar, amphibians, primates etc. | Infected by B. coli may result in acute in intestinal perforation or fulminating haemorrhagic dysentery and shock | The parasite is mostly transmits through cattle’s, contaminated water and unwashed vegetables | (Giarratana, Nalbone, Napoli, Lanzo, & Panebianco, 2021; Schuster & Ramirez-Avila, 2008) |
Opisthorchis spp. | O. viverrini and O. felineus are found among fish eating mammals | Symptoms are mostly asymptomatic. Heavier infections may be associated with nonspecific epigastric, nausea, anorexia and diarrhea, liver enlargement, bile stone etc. | They are mostly acquired by ingesting raw or undercooked freshwater fish or crab harboring metacercariae, aquatic snail etc. |
While previous researches were based on understanding the interaction of host-microbe and its portent (Marathe, Goel, Ranade, Jog, & Watve, 2002; Ruth et al., 2008), recent studies have shifted the focus more on zoonotic diseases and development of evidence-based risk assessment of humans and animals as a whole (Gibb et al., 2020; Keatts et al., 2021; Nath et al., 2021; Plowright et al., 2021).
Our study is the first such attempt to record the prevalence of gastrointestinal parasites among carnivores of DTR, the largest protected area of the state. The study reports gastrointestinal helminth parasites i.e. trematodes, cestodes and nematodes as more prevalent than protozoa in carnivores of the reserve. The species identified in the study were also previously reported from wild carnivores (Acharjyo, 2004; Beltrame et al., 2018; Colon et al., 2013; Figueiredo et al., 2021; Wicker et al., 2017), except Eimeria spp., Isospora spp. and Opisthorchis spp. that are reported to occur mostly in domesticated animal species (cattles, dog and cat) (Nath et al., 2021; Sarvi et al., 2018; Sevá et al., 2018). This may be due to cross-transmission from domestic animals as carnivores like Clouded leopard (Neofelis nebulosa), Leopard cat (Prionailurus bengalensis), Dhole (Cuon alpinus) and Golden jackal (Canis aureus) are known to feed on poultry, cattles and their intestinal remains in the region (Per. obs). Water bodies and crop fields in the vicinity of DTR also attract various carnivores like crab-eating mongoose (Herpestes urva), leopard cat (Prionailurus bengalensis), civets etc. and in the process may serve as potential sources of contaminants (faeces matters) (Gouda et al., 2020). The locals rely directly on the natural water streams flowing through the reserve for drinking and other activities, which may be another important factor for the spread of zoonoses in humans and cattle. The dependency of the local population (21%) on wild animals as sources of bushmeat and as ethnomedicines (Solanki, Lalchhandama, & Lalnunpuii, 2016) also increases the possibilities of cross-transmission to several folds. Agricultural fields in vicinities of protected areas had served as peri-habitation for several species of small carnivores, and their susceptibility for zoonotic diseases are high on possibilities. With the increase in landscape alteration, movement of people to wilderness areas, shrinking of wildlife habitats, free-ranging animals and seasonal trends, it is a matter of time when zoonotic parasites will break the nexus and find a new host in form of livestock and ultimately prevail among humans (Gibb et al., 2020; Gibb, Franklinos, Redding, & Jones, 2020; Keatts et al., 2021; Otranto et al., 2015; Plowright et al., 2021).
CONCLUSION
Parasite diversity in mammals has long served as important sources of information on diet-micro-biome relationship, anthropogenic food, the impact of alteration of land use pattern etc. However, as zoonoses from wildlife are emerging, the focus had shifted towards a meta-organism conservation approach. The study shows that the overall infection rate with gastrointestinal parasites from the sample collected is 90.47 %, which is exceptionally high. Several infectious pathogens were recorded in the study such as Spirometra spp., Paragonimus spp., Capillaria spp., Toxocara spp., A. lumbricoides and Opisthorchis spp., that can lead to serious health aliments or disorder if remains unchecked. These parasites are also a threat to the domestic animals that are important assets for the livelihood of locals around DTR. With ever-increasing interaction of human-wildlife in the form of wild bushmeat, under-processed water, and frequent visits to the protected area, the risk of parasitic spillover are at all-time high. As our study is based only on morphological identification, it is further necessary that a long term epidemiological study of parasites be undertaken, so as to understand the parasitism and prevent possible recurrence of existing infection in wild animals and cross transmission to humans.