Introduction
Acute kidney injury, characterized by acute loss of renal function, can occur due to multifactorial origins, such as exposure to toxic substances, metabolic disorders, and the prolonged use of nephrotoxic medications (Gameiro et al., 2020). It has become a global concern since the its prevalence steadily rising. In 2023 alone, the incidence of AKI achieved a staggering 13.3 million cases worldwide (Batte et al., 2023). This condition severely impacts patients' life expectancy and quality of life, as the kidneys play a crucial role in excreting metabolic waste and maintaining fluid and electrolyte balance.
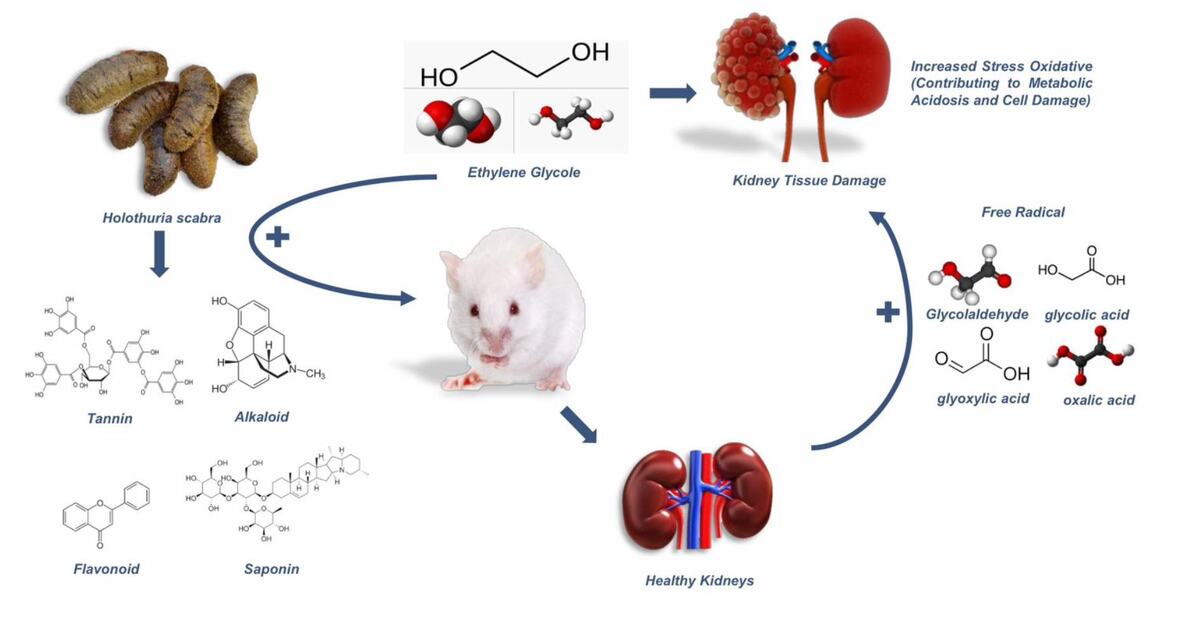
Among the causes of kidney damage, exposure to nephrotoxic compounds like ethylene glycol (EG) is particularly concerning. EG is widely used as industrial chemicals, particularly as anti-freezing agent. It has been recognized as a potent agent causing acute kidney injury, that mostly require hemodialysis to recover renal function. In 2023, a sudden increase in acute kidney injury (AKI) cases linked to ethylene glycol poisoning was reported in Indonesia and Gambia (Umar et al., 2023).
EG indigestion leads to the accumulation of its acidic metabolites, such as glycolic, glyoxalic, and oxalic acids (Hovda et al., 2017), which causes a non-respiratory acidosis characterized by a significantly elevated anion gap (Ghannoum et al., 2023). As EG is ultimately metabolized to oxalic acid, it forms calcium oxalate monohydrate crystals trapped in the renal tubules and cause significant damage (Pomara et al., 2008). Additionally, the metabolites trigger oxidative stress, inflammation, and kidney cell apoptosis. This cascade of events disrupts kidney function and can culminate in kidney failure (Liberek et al., 2013).
Antioxidants are critical in counteracting the oxidative stress induced by nephrotoxic substances like EG. When endogenous antioxidant defenses are overwhelmed, supplementation with external antioxidants becomes necessary. One of promising natural remedies is the Sandfish (Holothuria scabra), a species of sea cucumber, abundantly found in Asia waters. Bioactive compounds in Sandfish, such as flavonoids, tannins, saponins, polyphenols, and terpenoids, possess potent antioxidant properties (Hossain et al., 2022). In addition, its potent anti-inflammatory and antioxidant properties not only support overall health but also offer targeted protection for vital organs (Wargasetia et al., 2023). Therefore, this study aimed to explore the therapeutic effects of Sandfish extract on ethylene glycol-induced kidney injury in rats. The findings may inform future efforts to prevent and manage nephrotoxicity caused by environmental and chemical exposures, offering hope for improved renal health.
Materials and Methods
Materials
Sandfish (Holothuria scabra) were sourced from the Center for Environmental Education Puntondo, Takalar Regency, South Sulawesi, Indonesia (GPS coordinates -5.5880373 latitude and 119.4841993 longitude). Species identification was verified at the Laboratory of Zoology, Department of Biology, Hasanuddin University. Ethylene glycol (Emplura®) and 96% ethanol were purchased from a local chemical distributor in Makassar, Indonesia.
Sandfish Extraction
The Sandfish were processed by cutting into small pieces, drying using a food dehydrator, and extracting using the maceration method with 96% ethanol (1:2 w/v). The mixture was covered with aluminum foil and left at room temperature for 24 hours. This process was repeated three times with solvent replacement every 24 hours. The filtrate was pooled and concentrated using a vacuum rotary evaporator at 60°C and 80–150 rpm until a thick extract was obtained.
Phytochemical analysis
Qualitative analysis
The qualitative phytochemical screening of the Sandfish extract was performed using thin-layer chromatography (TLC) and specific spray reagents. The presence of alkaloids was identified by treating the TLC plate with freshly prepared Dragendorff’s reagent, resulting in an orange-brown coloration indicative of alkaloids. Flavonoids were detected using the boric acid method, with a greenish-yellow color change confirming their presence. Tannins were identified using Ferric Chloride (FeCl3) reagent, which produced a blue-black coloration (Sureshkumar, 2021). Triterpenoids were confirmed by the appearance of a red-violet stain upon treatment with Liebermann-Burchard's reagent. Saponins were tested in a test tube by diluting the extract in water; the formation of a stable foam after vigorous shaking indicated the presence of saponins (Godlewska et al., 2022).
Gas chromatography mass spectrometry (GC-MS) analysis
The phytochemical quantitative analysis was performed using a gas chromatography mass spectrometry (GC-MS). The chemical composition of Sandfish extract was determined based on a method previously outlined (Djabir et al., 2023), utilizing a Thermo Scientific Trace 1310 gas chromatograph paired with a TSQ 8000 Evo mass spectrometer (Mundelein, IL, USA).
Phenolic content assay
The total phenolic content of the Sandfish extract was determined quantitatively using the Folin Ciocalteu methods, with Gallic acid as the standard as explained in previous study by Adesina et.al. The result was expressed as Gallic Acid Equivalent (GAE) (Adesina et al., 2024).
Experimental Design
Animal Preparation
Male Wistar rats (age 3-4 months, 150-200 g, n=25) were obtained from the Pharmacology and Toxicology Laboratory, Faculty of Pharmacy, Hasanuddin University. The animals were housed under standard laboratory conditions (25°C, controlled humidity, 12-hour light/dark cycles) and provided with food and water ad libitum. All procedures involving animals adhered to international ethical guidelines for laboratory animals and were approved by the Health Research Ethics Committee of the Faculty of Medicine, Hasanuddin University (Approval No.: 579/UN4.6.4.5.31/PP36/2024).
Research groups
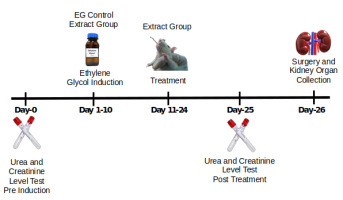
The experimental design is illustrated in Figure 1. Twenty-five rats were randomly assigned to five groups (n = 5/group):
- Healthy Control (HC): No ethylene glycol (EG) or Sandfish extract (SE) treatment; received distilled water.
- EG Control (EG): Treated with 30% EG solution (2 mL/day, orally) for 10 days but no SE treatment; received distilled water.
- EG + SE 500: Treated with EG for 10 days, followed by SE at 500 mg/kg for 14 days.
- EG + SE 1000: Treated with EG for 10 days, followed by SE at 1000 mg/kg for 14 days.
- EG + SE 1500: Treated with EG for 10 days, followed by SE at 1500 mg/kg for 14 days.
Blood Collection and Renal Function Test
Blood samples were collected from the retro-orbital plexus under intraperitoneal ketamine anesthesia. Samples were taken pre-induction (before EG administration) and post-treatment (after 14 days of SE administration). Approximately 3 mL of blood was collected using a capillary tube into vacutainers, centrifuged at 3000 rpm for 15 minutes, and the serum stored at 4°C until analysis.
Urea and creatinine levels were analyzed using the HumaLyzer 4000 (HUMAN, Germany). Measurements were performed twice: prior to ethylene glycol induction and after treatment with the sandfish extract. The measurement was performed based on the kits’ instruction (Human Diagnostic World, Germany). For urea levels, serum (10 µL) was mixed with 1000 µL (1 mL) of Reagent 1, and the mixture was incubated at 37°C for 3 minutes. Subsequently, 1000 µL (1 mL) of Reagent 2 was added, incubated for 5 minutes at 37°C. While for creatinine levels, the working reagent was prepared by mixing picric acid and sodium hydroxide (alkali) at a ratio of 1:4. A serum sample (100 µL) was then combined with 1 mL of the working reagent incubated internally in the HumaLyzer 4000 for 90 seconds at 37°C. The absorbance was measured at 340 nm (Lestiariani et al., 2023). The results were displayed on the device and expressed in mg/dL.
Evaluation of kidney relative weight
Following blood collection, the rats were euthanized, and their kidneys were carefully excised through laparotomy. The kidneys were then cleaned to remove any residual tissue or debris. Morphological evaluation of the kidneys was conducted to assess any structural abnormalities. The relative kidney weight was calculated from the organ’s weight divided by the rat's body weight and multiplied by 100, as described by previous study (Hasan et al., 2024).
Histopathological Analysis
Kidneys were fixed in 10% formalin buffered to pH 7.0–7.4 for 48 hours, dehydrated in graded ethanol, cleared, and embedded in paraffin. Sections (5 µm) were cut using a microtome and stained with Hematoxylin and Eosin (H&E) (Abd & Saeed, 2023).
Photomicrographs were captured from three selected fields in each kidney section at magnifications of 100×. Histopathological alterations, including inflammatory cell infiltration, Bowman's capsule dilation, tubular hemorrhage, tubular degeneration caused by oxalate crystals or protein casts, were evaluated by two independent observers. The microanatomical structure of the kidney was analyzed semi-quantitatively and scored based on the degree of damage (Table 1). The average percentage of damage was calculated using ImageJ software.
Statistical analysis
Biomarker data were expressed as mean ± standard deviation (SD). Statistical analyses were performed using SPSS software (version 30.0.0, IBM Corp., Armonk, NY, USA). Normality of the data was assessed using the Shapiro-Wilk test. For normally distributed data, comparisons between groups were conducted using one-way analysis of variance (ANOVA), followed by Tukey’s Honest Significant Difference (HSD) post-hoc test to identify pairwise differences.
Non-normally distributed data, including histopathological scoring, were analyzed using the Kruskal-Wallis test. Significant differences identified by the Kruskal-Wallis test were further examined using the Mann-Whitney U test to compare specific groups. A p-value < 0.05 was considered statistically significant.
Result
The yield of Sandfish extract
Fresh Sandfish weighing 3,600 g was extracted using 96% ethanol, yielding 68.4 g of thick extract, which corresponds to an extract yield of 22.3%.
Identification of phytochemical in Sandfish extract
Table 2
| Phytochemicals | Flavonoids | Tannins | Alkaloids | Triterpenoids | Saponins |
|---|---|---|---|---|---|
| + | + | + | - | + |
Table 2 presents the results of the qualitative phytochemical test of the Sandfish extract which indicate the presence of flavonoids, tannins, alkaloids, and saponins in the Sandfish extract, while triterpenoids were not found.
Chemical composition of Sandfish extract
The GC-MS analysis of the Sandfish extract identified multiple bioactive compounds, including fatty acids, esters, and sterols. Key components include (6Z)-Nonen-1-ol, l-Gala-l-ido-octose, Hexadecanoic acid, methyl ester, Cholestan-3-ol, (3. alpha.,5. beta.)-, and 2- Chloropropionic acid, decyl ester, among others. These compounds indicate potential pharmacological and nutritional properties of the extract (see Table 3.)
Table 3
| No. | Retention time | % Area | Compound name | Classification | Bioactivity (References) |
|---|---|---|---|---|---|
| 1 | 4.087 | 2.85 | l-Gala-l-ido-octose | Monosaccharides | Neuritogenic, Anti-hyper cholesteromia (El-Amier et al., 2023; Karthik et al., 2023) |
| 2 | 6.258 | 1.09 | Butanoic acid,3-methyl- | Fatty acid, | Antibacterial (Budayatin et al., 2021) |
| 3 | 6.341 | 2.15 | dl-Serine | Amino acid | (Wu et al., 2022) |
| 4 | 12.012 | 7.23 | (6Z)-Nonen-1-ol | Alcohol | (Devi, Muthu, 2015) |
| 5 | 12.843 | 1.12 | Razoxane | Alkaloid | Antibacterial (Ahmed et al., 2024) |
| 6 | 13.356 | 2.21 | 2-Chloropropionic acid,decyl ester | Fatty acid ester | Antibacterial (Karthik et al., 2023) |
| 7 | 20.478 | 1.04 | 2-Bromopropionic acid,pentadecyl ester | Fatty acid ester | Antibacterial (Mohan et al., 2020) |
| 8 | 20.776 | 2.39 | 7-Hexadecenoic acid,methyl ester, (Z)- | Fatty acid ester | Antioxidant (Karthik et al., 2023) |
| 9 | 21.144 | 2.75 | Hexadecanoic acid,methyl ester | Fatty acid ester | Antimicrobial (Shaaban et al., 2021) |
| 10 | 24.897 | 1.61 | 1-Heneicosanol | Alcohol | Antimicrobial, antioxidant, anti-inflammatory (Goda et al., 2020) |
| 11 | 25.274 | 1.34 | trans-13-Octadecenoic acid,methyl ester | Fatty acid ester | Anti-inflammatory |
| 12 | 25.749 | 1.60 | Tridecanoic acid,12-methyl-,methyl ester | Fatty acid ester | |
| 13 | 30.868 | 1.12 | Carbonic acid,eicosyl prop-1-en-2-yl ester | Fatty acid ester | |
| 14 | 34.649 | 1.35 | Methyl 3-hydroxytetradecanoate | Fatty acid | Antioxidant (Kücük, Yusufoğlu, 2013) |
| 15 | 34.897 | 1.04 | 2-Methyltetracosane | Alkanes | Free radical scavenger (Ramya et al., 2015) |
| 16 | 37.002 | 1.34 | 24-Norursa-3,12-diene | ||
| 17 | 38.375 | 1.37 | Hexadeca-2,6,10,14-tetraen-1-ol, 3,7,11,16 tetramethyl- | Antimicrobial, diuretic, anti-inflammatory (Adeniyi et al., 2024) | |
| 18 | 38.986 | 2.41 | Cholestan-3-ol, (3.alpha.,5.beta.)- | Steroid | (Volkman, 2006) |
| 19 | 39.755 | 1.70 | (1R,4aR,5S)-5-[(E)-5-Hydroxy-3-methylpent-3-enyl]-1,4a-dimethyl-6-methylidene-3,4,5,7,8,8a | Cyclic aldehyde | (Belov et al., 2023) |
The phenolic content
The total phenolic content of Sandfish extract was calculated using a standard curve of Gallic acid standard which obtain the value of 1.232 mg GAE/gr extract.
Serum biomarkers for renal function test
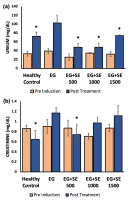
During pre-induction, the serum urea levels of all groups were not significantly different ranging from 25.26 ± 1.39 to 39.32 ± 6.29 mg/dl. After 10 days of ethylene glycol administration, followed by treatments for 14 days, a significant difference was found. In the EG group that only treated with water, the urea levels were almost tripled the initial level (p<0.05). However, all groups that were treated with Sandfish extract, at any given dose, did not experience a significant rise in their urea levels, and significantly lower than the EG group (Figure 2a). Apparently, treatment with a higher dose (1500 mg/kg) of Sandfish extract led to a smaller reduction of urea level compared to the dose of 500 mg/kg and 1000 mg/kg, but it was not significantly different from each other.
In addition, the creatinine levels of the rats treated with ethylene glycol without Sandfish extract treatment (EG group) increased significantly compared to the healthy controls (p<0.05). Unlike the urea levels, the creatinine levels of rats treated with Sandfish extract was more dose dependent. The lowest dose of 500 mg/kg led to a significant reduction of rats’ creatinine levels compared to the EG group (p<0.05). However, the 1000 mg/kg and 1500 mg/kg doses did not result in significantly lowered creatinine level (Figure 2b).
Kidney Relative Weight
Table 4
(*) p<0.05 compared to the healthy control.
Table 4 presents the body weight and both absolute and relative kidney weights of the rats. Among all groups, the two treatment groups, EG and EG + SE 1500, exhibited the highest absolute kidney weights. However, since the EG group had a lower body weight, it resulted in the highest relative kidney weight among all treatment groups. This increase in relative kidney weight was statistically significant compared to the healthy control group. These findings suggest that ethylene glycol (EG) treatment may influence renal tissue size and weight, potentially reflecting pathological changes induced by the treatment.
Histopathology of Rat Kidneys
Figure 3 depicts the micrographs of renal tissue for each treatment group showing a varying degree of histopathological changes. The healthy control group (Figure 3a) displayed minimal damage, including occasional hemorrhagic areas and inflammatory cell infiltration around the glomeruli. There was no evidence of Bowman’s capsule dilation or tubular degeneration caused by calcium oxalate crystals, as this group was not exposed to ethylene glycol.
In contrast, the ethylene glycol group (Figure 3b) exhibited extensive renal damage. This included widespread inflammatory cell infiltration, prominent dilation of Bowman’s capsule, severe tubular degeneration due to calcium oxalate crystal deposition, and extensive hemorrhage. The severity of these changes reflects the substantial kidney damage induced by ethylene glycol exposure, particularly in the tubular areas. While the EG group exhibited significant tubular damage due to calcium oxalate deposition, all rats survived the experimental period.
Figure 3
Representative histopathological micrographs of rat kidney tissues (H&E, 100×). (a) Healthy control with minimal histological changes; (b) Ethylene glycol (EG) control with severe renal injury; (c) EG + SE 500 with minimal damage; (d) EG + SE 1000 with moderate renal damage; (e) EG + SE 1500 with moderate renal damage. Observed histopathological changes observed: hemorrhage (blue arrow), tubular degeneration with calcium oxalate crystal (red arrow), congestion (orange arrow), Bowman’s capsule dilation (yellow arrow), inflammatory cell infiltration (green arrow), protein cast (white arrow).
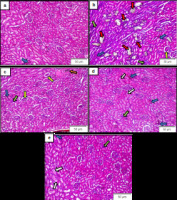
Treatment with Sandfish extract demonstrated varying levels of renal protection. In the EG+SE 500 mg group (Figure 3c), damage such as Bowman’s capsule irregularities, tubular hemorrhage, and inflammatory cell infiltration was still observed. However, tubular calcium oxalate crystals, which is the hallmark of EG-induced renal injury, were absent. The extent of damage in this group was markedly less severe compared to the EG group.
The EG+SE 1000 group (Figure 3d) showed no calcium oxalate crystals in the tubules. However, including hemorrhage and inflammatory cell infiltration was still evident. Unlike the EG+SE 500 group, protein accumulation in the tubules was found in this group. This may be associated with high dose of the Sandfish extract use in this study, potentially linked to its high protein content. In the EG+SE 1500 group (Figure 3e), the renal damage resembled that of the EG+HSE 1000 group. No calcium oxalate crystals were detected but protein accumulation in the tubules was evident.
Table 5
| Treatment Groups | Damage score |
|---|---|
| HC | 1.06 ± 0.14* |
| EG | 3.86 ± 0.18 |
| EG+SE 500 | 1.66 ± 0.24* |
| EG+SE 1000 | 2.07 ± 0.15* |
| EG+SE 1500 | 2.13 ± 0.18* |
(*) p<0.05 compared to the EG group.
The renal damage scores for different treatment groups are presented in Table 5. The healthy control (HC) group exhibited the lowest damage score (1.06 ± 0.14), indicating minimal renal damage. In contrast, the ethylene glycol (EG) group showed the highest damage score (3.86 ± 0.18), indicating severe renal injury. Treatment with sandfish extract (SE) at varying doses significantly reduced the damage scores compared to the EG group. The EG+SE 500 group had a damage score of 1.66 ± 0.24, followed by the EG+SE 1000 group with a score of 2.07 ± 0.15, and the EG+SE 1500 group with a score of 2.13 ± 0.18. These findings suggest a dose-dependent protective effect of sandfish extract, with the most substantial reduction in renal damage observed at the 500 mg/kg dose.
Discussion
Sandfish (Holothuria scabra) are highly valued for their numerous health benefits, attributed to their rich nutrient content. Historically, Sandfish have been renowned as tonics and medicinal remedies, especially in Asian countries, used to treat conditions such as hypertension, asthma, rheumatism, wounds, and burns (Bordbar et al., 2011). Extensive research on the biological and pharmacological activities of various sea cucumber species has highlighted their potential as anti-inflammatory agents (Ereguero et al., 2023; Feng et al., 2024). Its biological compounds such as saponins, flavonoids, and fatty acids have been identified as key contributors to these effects (Dewi et al., 2025; Feng et al., 2024; Puspitasari et al., 2023). Consistent with these findings, this present study also detected the presence of these compound groups in the Sandfish extract originated from South Sulawesi, Indonesia.
The composition of bioactive compounds in Sandfish is influenced by various factors, including the geographical location of sample collection. Environmental conditions such as substrate type, salinity, and overall ecological settings at the collection site significantly impact their chemical composition (Gamage et al., 2021). Previous studies have shown that Sandfish collected from different locations exhibit variations in the content of their biochemical profiles (Xu et al., 2018).
The GC-MS analysis of Sandfish extract has revealed its rich chemical profile. Key compounds identified was various, predominantly fatty acids and its esters, alcohols, steroids, and carbohydrates. These bioactive components are known for their pharmacological properties, such as anti-inflammatory, antioxidant, antimicrobials, and potential lipid-lowering effects (Hosseini et al., 2022). The presence of such compounds reinforces the potential of Sandfish extract as a promising source of nutraceutical and therapeutic agents.
This study investigated the protective effects of Sandfish extract against ethylene glycol-induced acute kidney injury. Renal dysfunction was observed in the ethylene glycol (EG) group, which received EG for 10 consecutive days without treatment. A significant increase in serum urea and creatinine levels (p<0.05) confirmed the presence of kidney injury. However, the administration of Sandfish extract effectively mitigated these effects. The Sandfish extract treatment significantly prevented the rise in urea levels at all doses tested, whereas a significant reduction in creatinine levels was observed only at the 500 mg/kg dose.
To further evaluate renal protection, morphological and histopathological analyses were performed. The relative kidney weight of EG-treated rats was significantly higher than that of healthy controls, indicating renal swelling or damage. Histopathological examination revealed extensive tubular degeneration, luminal oxalic acid crystal accumulation, hemorrhagic areas, and inflammatory cell infiltration in the kidneys of the EG group. Remarkably, treatment with Sandfish extract at 500 mg/kg completely prevented the formation of oxalic acid crystals, preserving the tubular architecture and preventing tubular damage. Although some glomerular dilation was observed, the overall kidney structure remained intact. Conversely, higher doses of Sandfish extract (1000 and 1500 mg/kg) also inhibited oxalic acid crystal accumulation but led to the presence of protein casts in some tubules. This phenomenon is likely attributable to the high protein content of Sandfish extract. These findings suggest that Sandfish extract, particularly at the 500 mg/kg dose, exhibits a potent protective effect against ethylene glycol-induced renal injury by preventing crystal deposition and preserving kidney morphology. However, higher doses may introduce additional protein-related effects, warranting further investigation into optimal dosing and long-term safety.
Ethylene glycol toxicity leads to significant kidney damage through the generation of toxic metabolites and oxidative stress. Once ingested, ethylene glycol is rapidly absorbed into the bloodstream and metabolized in the liver to form glycolic acid and oxalic acid. These metabolites are highly nephrotoxic and can cause cellular damage, metabolic disturbances, and the formation of calcium oxalate crystals, which deposit in the kidneys and impair their function (McMartin, 2009). Glycolic and oxalic acid directly damage kidney cells, disrupting their function and triggering cell death. This damage initiates an inflammatory response, where immune cells such as macrophages and neutrophils release pro-inflammatory cytokines, exacerbating tissue injury. Inflammation increases vascular permeability, leading to plasma leakage and hemorrhage into kidney tissues. Additionally, endothelial damage caused by these metabolites further contributes to bleeding and tissue injury by disrupting the coagulation system (Uy, Molina, 2023).
The metabolism of ethylene glycol also generates free radicals and reactive oxygen species (ROS), which amplify oxidative stress (Kadiiska, Mason, 2000). ROS damage critical cellular components like lipids, proteins, and DNA, further impairing kidney function. Oxalic acid, in particular, crystallizes with calcium to form insoluble calcium oxalate, creating sharp, needle-like structures that injure kidney tissues (Ermer et al., 2016). Moreover, the toxic effects of oxalic acid may extend beyond renal injury, potentially causing damage to other vital organs, including lungs and heart, which may lead to fatal outcomes (Furnica et al., 2017).
The nephroprotective effect of Sandfish Extract is believed strongly related to its antioxidant and anti-inflammatory action (Wargasetia et al., 2023). Antioxidant compounds in Sandfish extract may act by stabilizing free radicals generated by ethylene glycol metabolism, which in turn interrupt oxidative chain reactions. Flavonoids, among the strongest antioxidant compounds in Sandfish, exhibit dual antioxidant and anti-inflammatory activities (Wulandari et al., 2022). By scavenging superoxide and lipid peroxyl radicals, flavonoids prevent oxidative damage to biomolecules and reduce oxidative stress (Li et al., 2020). Their anti-inflammatory properties are linked to the inhibition of leukocyte adhesion to endothelial walls, decreasing leukocyte immobilization, and reducing the production of pro-inflammatory cytokines and complement factors (Werner et al., 2015).
Tannins, also present in Sandfish, contribute to renal protection by mitigating oxidative stress and promoting the cellular regeneration (Peng et al., 2022). Tannins delay oxidation processes, protecting tissues and organs from damage caused by free radicals. Under oxidative stress conditions, when endogenous antioxidant levels are depleted, tannins help restore balance by preventing the accumulation of unbound free radicals (Soldado et al., 2021), and thereby, may protect renal proximal tubule cells from apoptosis initiated by oxidative stress.
Saponins in Sandfish further support kidney health by inhibiting vascular permeability, limiting inflammation (Avunduk, 2024), and may act as superoxide scavengers by forming hydroperoxide intermediates (Lina et al., 2024). This reduces biomolecular damage caused by free radicals. Saponins also exhibit anti-inflammatory effects by suppressing cyclooxygenase-2 (COX-2) activity (Wijesekara et al., 2024), thereby reducing the production of prostaglandins, which are key inflammatory mediators. Additionally, saponins suppress the expression of inflammatory biomarkers such as TNF-α and interleukin-6 in macrophages stimulated by lipopolysaccharides (LPS), further demonstrating their anti-inflammatory efficacy (Zou et al., 2017). These antioxidant and anti-inflammatory properties of Sandfish-derived chemical components supports their potential as a promising novel nephroprotective agent.
Conclusion
In conclusion, this study confirmed the nephroprotective potential of Sandfish (Holothuria scabra) extract against ethylene glycol-induced kidney damage, demonstrated by improved renal biomarker levels and enhanced renal histological structures. The qualitative analysis of the extract identified key groups of bioactive compounds, including alkaloids, flavonoids, tannins, and saponins, while the GC-MS analysis revealed significant components such as fatty acids, alcohols, carbohydrates, and sterols. These bioactive compounds are known for their antioxidant and anti-inflammatory properties, which likely contribute to the observed protective effects of the Sandfish extract.
Limitation
This study has some limitations. First, the exact mechanisms through which individual bioactive compounds exert their protective effects remain unclear. Second, the study did not assess the long-term safety or efficacy of Sandfish extract administration. Additionally, the findings suggest that the optimal dose of Sandfish extract in this study was 500 mg/kg, while higher doses may have adverse effects, such as protein cast formation in renal tubules. The urea levels in sandfish-treated rats at doses of 500 mg/kg and 1000 mg/kg were significantly lower compared to the healthy controls. However, we did not conduct a dedicated toxicity study in this experiment. Previous research has indicated that Holothurians extract does not cause mortality or any sign of toxicity at a dose of 2000 mg/kg (Fahmy et al., 2015). This suggests that the doses used in our study are within a safe range. Nevertheless, future studies should include a more comprehensive toxicity assessment to strengthen the findings and address this concern, exploring long-term safety and efficacy, and optimizing dosing strategies to fully harness the therapeutic potential of Sandfish extract.