Introduction
EPS which are produced by microorganisms, are high-molecular-weight natural biopolymers composed of several kinds of sugar residues. Depending on the effect of ecological conditions, the structure and functional performance of microbial EPS might vary. For growth and development, several bacterial strains have the capacity to generate EPS. Characterization of EPS from Lactobacillus strains in cucumber and cabbage to assess the capacity for production (Singh, Saini, Puranik, Gupta, & Dubey, 2016). Numerous thermophilic bacteria, in addition to archaea, are efficient producers of significant quantities of EPS for industrial use. Potential bacterial strains from harsh climatic settings, such as Bacillus thermantarcticus, Geobacillus thermodenitrificans, and Bacillus licheniformis, have been identified and described for a variety of purposes. (Kambourova et al., 2009).
Many halophilic strains from Archaea and marine sediments, such as Haloferax, Haloarcula, Halococcus, Natronococcus, and Halobacterium, have been examined for their ability to produce EPS, according to some previous findings. The isolated bacterial strains were tested for EPS generation in the appropriate medium. Then, FTIR, HPLC, and GC-MS analyses were used to describe the screened bacterial strains. The instrumentation analysis was performed to look for different groups and sugar residues. (Shankar et al., 2021).
Since most EPS was produced during the log phase of the bacterium, the bacterial growth phase can generally have an impact on the production of EPS. Additionally, the main factor in bacterial development at the time of yield is the composition of the medium, namely the presence of carbon, nitrogen, and other mineral sources. Therefore, environmental factors like temperature and pH are crucial for the generation of yield. In addition, because they require a suitable quantity to consume food material in the medium, inoculum concentration usage is another important component of EPS output (Vuyst, Degeest, & B, 1999).Maheswari, Arjun, Sankaralingam, and Sivakumar (2020) studied the capacity of microbes to produce EPS. The bacterial strain that produces EPS were isolated from a marine source and identified using biochemical and genomic sequencing techniques. By using the aforementioned approaches, the candidate isolates was identified as Bacillus sp. As a result, the current work was carried out to maximise the EPS generation from the marine isolate of Citrobacter sp.
Materials and methods
Screening of bacterial strain
To test the generation of EPS, the chosen isolates were screened on Zobell marine agar. The bacteria were streaked on agar and kept at 37°C for 72 hours. The development of ropy or mucus discharge after incubation is a sign of success. The massive producer was then picked for more experimental investigation.
Optimization of cultural conditions for EPS production
The chosen bacterial isolates underwent purification and characterisation procedures after being tested for EPS production. After 72-hour incubations, the ambient factors including pH and temperature were assessed for the EPS-producing capacity.
Effect of different pH on bacterial growth and EPS production
After a 72-hour incubation period, it was established how different pH levels affected EPS production. To find out how different pH values, including 2, 3, 4, 5, 6, 7, 8, and 10, affected the formation of EPS, the production medium was changed. An optical density measurement at 600 nm was used to gauge the growth of the chosen bacterial strains.
Effect of different temperatures on bacterial growth and EPS production
After a 72-hour incubation period, the impact of various temperatures on EPS generation was assessed. To test the impact of temperature on EPS yield, a production medium made at different temperatures (20, 30, 40, and 50oC) was employed.
Effect of different incubation times on bacterial growth and EPS production
After 72 hours of incubation, the impact of varied incubation periods on EPS production was assessed. To investigate the impact of incubation time on EPS yield, the production medium of a chosen bacterial culture was incubated at various incubation times, including 24, 48, 72, 96, and 120 hours.
Effect of different carbon sources on bacterial growth and EPS production
After a 72-hour incubation period, the impact of various carbon sources on EPS synthesis was assessed. To evaluate the impact of carbon dosage on EPS yield, several carbon sources including arabinose, glucose, galactose, maltose, xylose, fructose, mannitol, and lactose were added to the production medium at 1% concentration.
Effect of organic and inorganic nitrogen sources on bacterial growth and EPS yield
After a 72-hour incubation period, the impact of various organic and inorganic nitrogen sources on the formation of EPS was examined. To investigate the impact of nitrogen sources on microbial EPS yield, several nitrogen sources including peptone, yeast extract, raseine, glycine, ammonium nitrate, potassium nitrate, ammonium sulphate, sodium nitrate, and urea were added to the production medium at 1% concentration.
Effect of metal ions on bacterial growth and EPS production
After a 72-hour incubation period, the impact of various metal ions on EPS formation was examined. To evaluate the impact of metal ions on EPS yield, various metal ions including calcium chloride, copper sulphate, zinc sulphate, ferrous sulphate, ferric chloride, manganese sulphate, magnesium sulphate, and lead acetate were added to the production medium at 1% concentration.
Effects of amino acids on bacterial growth and EPS production
After a 72-hour incubation period, the impact of various amino acids on EPS generation was examined. To investigate the impact of different amino acids on EPS production, the production medium was individually supplemented with each of the following amino acids at a concentration of 0.1%: Glycine, Glutamine, Cysteine, Alanine, Methionine, Aspartic acid, Ornithine, Proline, Threonine, Phenylalanine, Serine, Leucine, Hydroxy proline, 2-aminobutyric acid, Norleucine, Argin.
Effects of surfactants on bacterial growth and EPS production
After a 72-hour incubation period, the impact of various surfactants on EPS production was determined. To examine the impact of various surfactants on microbial growth and EPS production, 0.2% concentrations of PEG, Tween 80, Tween 20, CTAB, and Triton X 100 were individually added to the production medium.
Effects of NaCl concentration on bacterial growth
After a 72-hour incubation period, the impact of varying sodium chloride concentrations on EPS generation was studied. To explore how sodium chloride affects EPS synthesis, various NaCl concentrations, including 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5%, were added to the production medium.
Effect of inoculum concentrations on bacterial growth and EPS-production
After 72 hours of incubation, the impact of different culture inoculum concentrations on EPS generation was assessed. To investigate how inoculum concentrations affected EPS production, various inoculum concentrations including 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5 were individually added to the production medium.
Effect of Incubation time in hours on bacterial growth and EPS production
After 72 hours of incubation, the impact of varied incubation periods on EPS generation was assessed. To explore the impact of incubation time on EPS production, various incubation times, including 24 hours, 48 hours, 72 hours, 96 hours, and 120 hours, were added to the production medium. The optical density was measured at 600 nm to gauge the growth of the organism.
RESULTS
SCREENING OF EPS -PRODUCING BACTERIA
Isolation and selection of EPS-producing bacteria
Rameshwaram district were collected to examine the soil's capacity to produce EPS. For EPS production screening, the soil samples were serially diluted and plate-tested. The culture plates were then incubated for 72 hours at 37C. The streaking bacterial strains had ropy look or glittering ability after incubation.
The dominating EPS producer was used for additional experimental study after the EPS production of the screened bacterial strains was assessed. The yield EPS on malt agar medium or ropy appearance was used to determine the maximum amount of EPS produced (Figure 1).
Morphological and biochemical characteristics of EPS-producing bacteria
The chosen strain was recognised as Citrobacter sp. in Bergey's handbook of Determinative Bacteriology. The outcomes of every carbon-utilizing and biochemical study have been obtained (Table 1).
Table 1
Biochemical test for the bacterial isolate
OPTIMIZATION OF CULTURAL CONDITIONS FOR THE PRODUCTION OF EPS
Then the screened bacterial strains were subjected to the optimization of culture conditions through various process parameters.
Effect of pH on EPS production
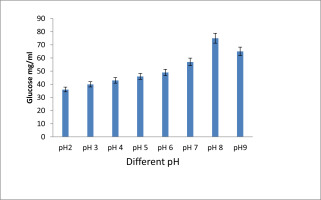
Figure 2 depicts how pH affects the candidate isolates ability to produce EPS. After 72 hours of incubation at 370C with different pH levels, the generation of EPS was measured. The pH at which the most EPS was produced was 8, while the pH at which the least was produced was 2. After that, due to too low and too high production levels being deadly to the bacterial strains, there was no discernible increase in output.
Effect of temperature on EPS production
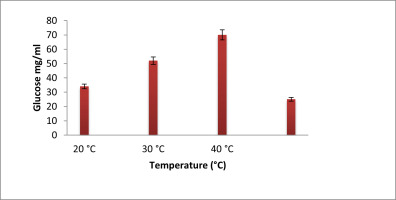
Figure 3 depicts how temperature affects the candidate isolates's ability to produce EPS. After 72 hours of incubation at various temperatures, the generation of EPS was measured. The highest level of EPS generation was seen at 400C among the tested temperatures. Contrarily, the minimal EPS production was noted at 200C, following which no appreciable production was noted.
Effect of carbon on EPS production
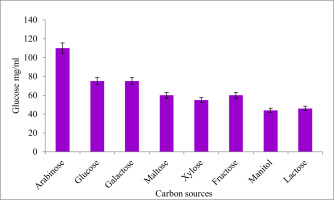
Figure 4 depicts how different carbon sources affect the candidate isolates's ability to produce EPS. Following a 72-hour incubation period at 37°C, the impact of carbon sources on Citrobacter sp. synthesis of EPS was assessed. The medium Xylose produced the least amount of EPS and the highest amount of EPS when arabinose served as the substrate.
Effect of nitrogen sources on EPS production
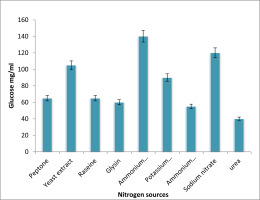
The impact of various organic and inorganic nitrogen sources was depicted in Figure 5. The impact of various nitrogen sources on the generation of EPS following a 72-hour incubation period at 37°C. The least quantity of EPS synthesis was seen among the sources evaluated on the 210 medium supplemented with urea, while the most were shown on the medium supplied with ammonium nitrate.
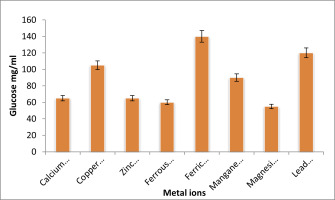
Effect of metal ions on EPS production
The impact of different metal ions on the synthesis of EPS is shown in Figure 6. After 72 hours of incubation at 370 C with varied metal ions concentrations, the generation of EPS was measured. The least quantity of EPS production among the investigated metal ions was seen in the medium with magnesium sulphate added. While the medium treated with calcium chloride showed the highest level of EPS generation.
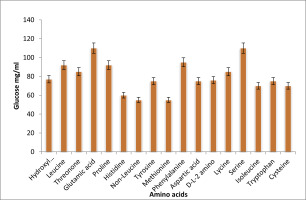
Effect amino acids on EPS production
Figure 7 depicts the impact of amino acids on the candidate isolates of Citrobacter sp EPS production. After 72 hours of incubation at 370 C with various amino acids, the production of EPS was measured. Serine-supplemented medium showed the highest level of EPS generation among the evaluated amino acids. The medium with methionine added showed the least quantity of EPS generation during the same period.
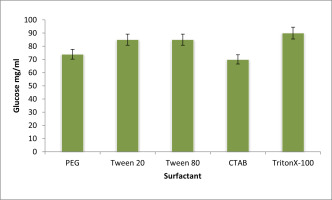
Effect of surfactants on EPS production
The impact of surfactants on the candidate isolates Citrobacter sp EPS` generation is shown in Figure 8. The results of testing several types of surfactants on the generation of EPS following a 72-hour incubation period at 37°C. In the medium with CTAB added, the least quantity of EPS generation among the evaluated surfactants of was seen. Triton X-100 acted as the medium when the maximum level of EPS generation was seen.
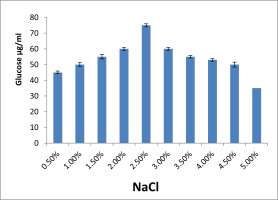
Effect of NaCl concentration on EPS production
The candidate isolates of Citrobacter sp. Influence`s of sodium chloride on EPS generation is shown in Figure 9 . After a 72-hour incubation period at 37°C, the impact of different sodium chloride concentrations on the synthesis of EPS was evaluated. The 2.5% NaCl added medium showed the highest yield among the tested concentrations, whereas the 5% NaCl added medium showed the least quantity of EPS generation.
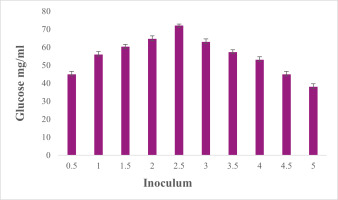
Effect of inoculum concentrations on EPS production
Figure 10 depicts the results of a study on the impact of different inoculum concentrations on EPS production. After 72 hours of incubation at 370 C with varying concentrations, the generation of EPS was measured. Among the investigated concentrations, 2.5 ml inoculums-given medium showed the highest quantity of EPS generation. The 5% inoculum concentration for EPS formation was considered to be the minimum for EPS production.
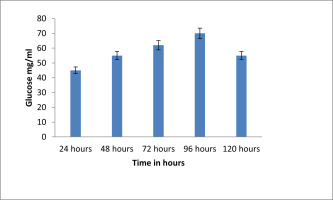
Effect of incubation time on EPS production
The potential isolate Citrobacter sp. and the impact of varied incubation times on EPS generation are shown in Figure 11 . After 72 hours of incubation at 370 C with varying incubation periods, the generation of EPS was measured. The incubation period of 96 hours produced the most EPS out of the investigated time periods. The least quantity of EPS generation, however, was seen after 24 hours of incubation.
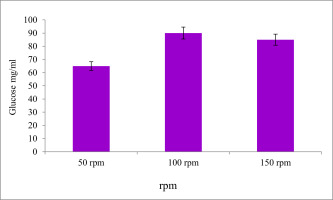
Effect of rpm on the EPS production
Figure 12 shows the effect of various rpm on EPS yield from the candidate isolates was determined. The minimum EPS production was viewed at 96 hours incubation period observed at 150 rpm and the maximum amount of EPS production was observed at 100 rpm (0.90 ± 0.1 mg/ml).
DISCUSSION
The largest sources of biological and chemical diversity are found in the oceans, seas, and lakes, which not only serve as a rich reservoir of untapped and/or unknown microorganisms but also as a great source of novel biomolecules with potential biotechnological applications in the pharmaceutical, medical, cosmetic, feed, food, and enzyme industries (Finore, Donato, Mastascusa, Nicolaus, & Poli, 2014; Satpute, Banat, Dhakephalkar, Banpurkar, & Chopade, 2010). These novel microbes are unique, which makes them excellent models for studying the environment and a source of new chemicals with commercial significance. EPS a byproduct of microorganisms, have important physiological roles and a range of practical uses (Finore et al., 2014). The complex macromolecular polyelectrolyte substance that makes up microbial EPS has a variety of molecular masses and can be either homopolymeric or heteropolymeric in nature. They have a variety of structural behaviours depending on the situation. They have unique ecological and physiological characteristics that serve as protection against adverse situations including desiccation and antimicrobial chemical substances (Shankar et al., 2021)
The complex macromolecular polyelectrolyte substance that makes up microbial EPS has a variety of molecular masses and can be either homopolymeric or heteropolymeric in nature. They have a variety of structural behaviours depending on the situation. They have unique ecological and physiological characteristics that serve as protection against adverse situations including desiccation and antimicrobial chemical substances. (Shankar et al., 2021).
Correspondingly,Maheswari et al. (2020) EPS-producing bacterial strain that was isolated from marine sand, and the culture conditions were assessed using a variety of process factors. SSankaralingam, Eswaran, Boomi, Sundaram, and Shankar (2014) also studied the growth-promoting bacterial strain isolated from the rhizosphere soil to improve soil fertility.
Maheshwari, Qais, Althubiani, Abulreesh, and Ahmad (2019) reported that Zobell agar medium, the synthesis of EPS from Bacillus sp. isolated in marine material and the hyper yield were evaluated. In the present study, the maximum production of EPS was observed from bacteria in Malt medium agar (0.88 ± 0.04 mg/ml). Likewise,Shankar et al. (2021) studied for industrial purposes, the synthesis of EPS from Lactobacillus species was examined in Zobell marine agar medium.
Kawai et al. (1992) reported that the pH at which the bacterial strain produced exopolysaccharides was discovered to be 7. In the present study, pH 7.0 was shown to be the ideal culture setting for the development of EPS. Correspondingly,Maheswari et al. (2020) studied the production of ESP from marine isolate Bacillus sp. was recorded in pH 7. Exopolysaccharides are complex macromolecular polyelectrolyte mixtures produced by Lactobacillus species vary in their molecular structure and behaviour depending on the environment. (Nwodo, Green, & Okoh, 2012; Shankar et al., 2021).
EPS of microbial origin are preferred over gums of plant origin since they are readily biodegradable and climate-susceptible, according to earlier references. They are an important material for pharmaceutical applications due to their rheological performance. (Shankar et al., 2021). In our study, the optimal yield was found at 40oC. In the same way,Maheshwari et al. (2019) reported that the production of EPS from Bacillus was possessed at 40⁰C. The marine bacterium Hahella chejuensis, which was found on Cheju Island, was used to conduct research on the impact of growing temperature on the formation of an EPS known as EPS-R. In the 20 to 25°C temperature range, productivity was at its maximum; beyond 30°C, production declined (Ko, Lee, Park, & Lee, 2000).
When nutrition sources like carbon and nitrogen are present, bacterial EPS operate significantly better, forming a biofilm that is used for colonisation. (Madhuri & Prabhakar, 2014; Maheshwari et al., 2019). Protective barriers as act as anti-microbial agents against various microorganisms (Burgula et al., 2007; ) (Maheswari et al., 2020). In the present investigation, the maximum yield was found to be arabinose as carbon sources added medium. The present work is more or less similar to the earlier report ofMaheshwari et al. (2019) who found that huge production was recorded in sucrose supplemented medium. In Zunongwangia profunda SM-A87, the impact of carbon sources was also investigated. The addition of lactose to the basic marine medium resulted in the highest amount of EPS synthesis, which was measured at roughly 6.47 g/L. Glucose, mannose, maltose, and sucrose were the other carbon sources examined (Liu et al., 2011).
Numerous researchers have looked into the anti- and antioxidant properties of EPS to meet industrial needs. To avoid the recurrent ingestion of hazardous chemical substances, the bioactive potential of bacterial EPS is, therefore, more important in the modern world (El-Newary, Ibrahim, Asker, Mahmoud, & Awady, 2017). In the present study, the maximum production of EPS was found to be 2.5% sodium chloride medium. Similarly,Maheswari et al. (2020) reported that the production of EPS from marine Bacillus sp. was possessed in a 2% supplemented medium.
CONCLUSION
The present study concludes the opportunity to the importance of marine soil bacteria possessing pharmacological activities. As the EPS from bacteria may have various pharmacological activities such as anticoagulant, antioxidant, anti-inflammatory, antimicrobial, antitumor, anti-complementary and anti-adhesive activities can also be used for various pharmaceutical applications.