Introduction
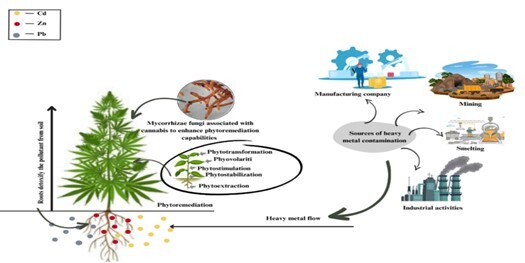
The insidious and harmful nature of heavy metals, which are hazardous pollutants with toxic effects on humans and the environment, is well-documented. These metals tend to build up in the soil as a result of various industrial processes including manufacturing, smelting, and mining (Banuelos & Ajwa, 1999; Placido & Lee, 2022). These metals provide a serious and immediate risk to human and environmental health when found in soil, with a focus on the extremely poisonous components of lead, cadmium, and nickel (Hou et al., 2020). These metals are so toxic that prolonged exposure, even at low concentrations, can seriously harm the kidneys, liver, and brain as well as raise the chance of acquiring serious illnesses including cancer. In light of these hazards, these heavy metals must be effectively monitored and managed to prevent their continued accumulation and potential for harm. This requires sustained vigilance and effective environmental management measures to mitigate the dangers associated with heavy metal pollution Garbisu and Alkorta (2001); Järup (2003).
A sustainable and economical way to clean up contaminated soil and water is through phytoremediation, which uses plants to absorb, detoxify, or immobilise toxins (Singh, Gautam, Mishra, & Gupta, 2011). This technique makes use of some plants' innate capacity to draw and hold onto contaminants from the soil, such as pesticides, heavy metals, and petroleum hydrocarbons. The contaminants can then be stored in the tissues of these plants or broken down into less hazardous molecules, leaving the soil and water safe and clean (Cunningham & Berti, 1993). Phytoremediation presents numerous advantages compared to conventional remediation techniques like excavation and chemical treatment. It stands out as a non-invasive, eco-friendly method that eliminates the need for hazardous chemicals or equipment. Additionally, phytoremediation boasts scalability, rendering it a practical choice for remediating polluted sites. An especially encouraging aspect of phytoremediation is its potential application in addressing the global challenge of heavy metal pollution. Certain plants, such as cannabis, are particularly effective in accumulating heavy metals in their tissues, making them useful candidates for phytoremediation (Raskin, Smith, & Salt, 1997).
Due to its association with recreational drug use, the cannabis plant, commonly referred to as marijuana, has been a subject of ongoing debate. Nevertheless, there has been a growing interest in exploring its potential applications in various industries, including food, textiles, and medicine. A particularly noteworthy area of focus is its remarkable ability to employ phytoremediation for the removal of heavy metal pollution from contaminated soil. In this context, the cannabis plant variant known as industrial hemp has emerged as a promising candidate for this crucial environmental purpose. Industrial hemp, a specific subtype of Cannabis sativa, is renowned for its robust and fibrous stalks, making it suitable for a range of commercial applications. Furthermore, research has indicated its exceptional efficacy in extracting heavy metals such as lead, cadmium, and nickel from polluted soil. Through the process of phytoremediation, industrial hemp can accumulate these heavy metals within its tissues, effectively detoxifying the soil from these hazardous contaminants (Placido & Lee, 2022).
Review Methodology
The methodology followed for the duration of this critical review is as follows (Figure 1).
Literature Review
This review study uses a comprehensive and all-inclusive technique, searching widely via reputable scientific databases such as PubMed, Scopus, and Google Scholar. Among the pertinent terms included in the search strategy are "industrial hemp," "phytoremediation," "heavy metals," "soil contamination," and "pollution." The aim of this methodology is to thoroughly collect and evaluate relevant concepts and scientific data about the applicability of industrial hemp for the phytoremediation of heavy metal-contaminated environments. The goal of the literature search is to locate and get studies that make a substantial contribution to our knowledge of industrial hemp's potential application in phytoremediation for the mitigation of heavy metal contamination.
Article Screening and Inclusion Criteria
The ascertained scholarly pieces were subjected to a screening process based on their pertinence to the subject matter, and only those that fulfilled the prescribed inclusion criteria were deemed worthy of further scrutiny. The chosen articles were meticulously evaluated to their experimental design, methodological framework, empirical findings, and ultimate conclusions.
Data Synthesis and Presentation
This review essay is structured in a methodical and orderly manner. The manuscript is divided into various sections that clarify the history and situation surrounding heavy metal pollution, emphasise the need for phytoremediation as a remediation technique, list the qualities of industrial hemp that make it a good fit for phytoremediation, and discuss the complex mechanisms controlling heavy metal uptake and detoxification in industrial hemp. The primary aim of this review paper is to provide a thorough examination of the potential of industrial hemp as a useful instrument for phytoremediation in heavy metal-contaminated soils.
Industrial Hemp as a Phytoremediator
The plant commonly referred to as industrial hemp, or simply hemp, is recognized for its rapid growth and has sparked interest as a potential phytoremediator owing to its ability to absorb heavy metals and other contaminants into its tissues. Hemp finds extensive use in industries such as paper, textile, and construction (Adesina, Bhowmik, Sharma, & Shahbazi, 2020; Hall, Bhattarai, & Midmore, 2012). Studies have indicated that hemp is effective in removing various heavy metals, including cadmium, lead, copper, and nickel, from contaminated soil. This efficacy is attributed to its deep root system, capable of reaching depths of up to six feet, allowing the plant to absorb and accumulate contaminants from deeper soil layers. Given its substantial biomass production, hemp emerges as a valuable tool in phytoremediation, facilitating the extraction of significant quantities of contaminants from polluted soil (Amaducci & Gusovius, 2010; Cherney & Small, 2016; Chundawat, Beckham, Himmel, & Dale, 2011). Research indicates that throughout a growing season, hemp may eliminate up to 50% of cadmium and 90% of lead from polluted soil. However, the amount of contaminants in the soil, how long it takes for it to develop, and other environmental conditions can all affect how successful hemp is as a phytoremediator (Hu & Lim, 2007). One possible aspect to take into account while using hemp in phytoremediation is how to properly dispose of contaminated plant material. Because the plant may store heavy metals and other contaminants in its tissues, it is essential to dispose of harvested plant material carefully to avoid unintentionally contaminating the environment. To fully explore hemp's potential in phytoremediation and to especially address issues with the safe and responsible disposal of polluted plant material, more study is necessary (Das et al., 2017; Zhao et al., 2020).
Table 1
Heavy-metal concentrations in hemp. Listed tissues represent those with the highest concentration of metal as reported from each of the studies (Placido & Lee, 2022)
Metal | Tissue | Concentration (mg kg−1) | References |
Zn | Flower Root Shoot | 78.6 5029.8 43.9 | (Angelova, Ivanova, Delibaltova, & Ivanov, 2004; Mongioví et al., 2021; Shi & Cai, 2010) |
Cu | Flower Root Shoot | 10.2 1530 29 | |
Cd | Flower Leaf Root | 1.22 0.38–23.2 1362 | |
Ni | Leaf Root | 1.5–123 13.6–321.8 | |
Cr | Root | 6.2–100 | |
Se | Shoot | 1300 |
Potential Industrial Application of Hemp in Phytoremediation and its Mechanism
The application of Hemp in phytoremediation may offer potential prospects for various industries (Table 1). For instance, contaminated cannabis crops could be harvested and utilized in the production of biofuels, thereby reducing the reliance on non-renewable energy sources and advancing ecological sustainability.
Chromium phytoremediation
Chromium phytoremediation refers to utilizing plants to eliminate or reduce the presence of contaminants or reducing the concentration of chromium in contaminated soil, water, or air (Sankhla & Kumar, 2019). Heavy metal chromium finds extensive application in industrial processes including cement manufacturing, leather tanning, and metal plating. However, both persons and the ecosystem may be harmed by exposure to excessive chromium concentrations (Kerkeb & Kramer, 2003; Rai, Lee, Zhang, Tsang, & Kim, 2019; Udawat & Singh, 2020). There are several methods for dealing with chromium contamination, such as phytotransformation, phytoextraction, and phytostabilization. Chromium is extracted by plants from polluted soil and stored in their tissues during the phytoextraction process. This procedure enables the plant material containing chromium to be collected later and disposed of properly (Verma, Sankhla, Jadhav, Parihar, & Awasthi, 2021). While large decreases in soil chromium concentrations may require many growing seasons, this approach is successful in eliminating high quantities of the mineral. Using plants to immobilise chromium in the soil and stop it from leaking into groundwater or being absorbed by plants is known as phytostabilization (Ent, Baker, Reeves, Pollard, & Schat, 2013; Placido & Lee, 2022; Rascio & Navari-Izzo, 2011). Lower chromium content soils are ideal for this approach. Utilisable plants include deep-rooted, high-biomass plants such as poplars and willows (Angelova et al., 2004). However, the effectiveness of phytoremediation can vary depending on the type and concentration of chromium, the plant species used, and environmental conditions. Therefore, careful selection of the appropriate plants and methods is essential for the successful phytoremediation of chromium-contaminated sites (Raimondi, Rodrigues, Maucieri, Borin, & Bona, 2020; Shi & Cai, 2010).
Zinc Phytoremediation
Zinc phytoremediation is the process of employing plants to remove or reduce toxins or lower the level of zinc in contaminated soil, water, or air. Heavy metals like zinc are usually found in industrial waste and mine sites. Zinc is important for plant development (Banuelos & Ajwa, 1999; Stonehouse et al., 2020). Nevertheless, both people and plants may be poisoned by excessive zinc concentrations. Zinc may be bioremediated by a variety of techniques, such as phytoextraction and phytostabilization. Through a process called phytoextraction, plants absorb zinc from polluted soil and store it in their tissues, which may then be collected and disposed of appropriately. While large decreases in zinc concentrations in soil may need many growing seasons, this strategy is successful in eliminating high quantities of zinc from soil (Liu & Tran, 2021). Using plants to immobilise zinc in the soil and stop it from evaporating into groundwater or being absorbed by plants is known as phytostabilization. This technique works best with soils that have lower zinc concentrations. Plants like willows and poplars that produce a lot of biomass and have deep root systems can be used to achieve this (Chalot, Blaudez, Rogaume, Provent, & Pascual, 2012). Because they can store large amounts of zinc in their tissues, plants like sunflowers and Indian mustard are useful in zinc phytoremediation. However, the kind and concentration of zinc, the type of plant species utilised, and the surrounding circumstances may all affect how successful phytoremediation is (Huang et al., 2018; Schreurs, Voets, & Thewys, 2011; Schröder et al., 2018).
Copper Phytoremediation
Hemp leaves tend to accumulate copper (Cu) more than fibres. The plant's major antioxidant enzymes, Glutathione-disulfide reductase (GSR) and phospholipase D-α (PLDα), offer protection against oxidative damage caused by reactive oxygen species (ROS) generated under metal-stress conditions. When hemp accumulates high concentrations of Cu, GSR and PLDα expression levels are induced (Baker, 1981; Ghosh & Singh, 2005). Aldo-keto reductase, an NAD(P)H-dependent enzyme, was found to increase in hemp grown under Cu stress, which plays a role in the detoxification process by enhancing the scavenging ability of the cell. The protein decreases the concentration of ions, thereby facilitating their interaction with other proteins, including phytochelatins that transport them to the vacuole. While the treatment of Cu did not affect photosynthesis, it led to a significant decrease in the aerial parts and root system architecture of the plant.
Table 2
Presents the measured concentrations of significant metals across different hemp tissues.
Variety | Cd | Pb | Cu | Zn | Ni | Cr | Location | References |
|
|
|
|
|
|
|
|
|
Armanca | 3.4 | 0 | 11.9 | 46–68 | 3.5 | 0.86 | Romania | |
Beniko | 36.5– 33457 | 2.99– 32084 | 28.2– 19022 | 70.1– 37440 |
|
| Czechia |
|
Bialobrzeskie | 0.37–0.84 | 1.6–9.13 |
| 7.98– 42.56 |
|
| France | |
| 0.29–4.3 |
|
|
|
|
| Poland | (Vaitsis et al., 2022) (Wielgusz, Praczyk, Irzykowska, & Świerk, 2022) |
| 31.2– 55678 | 2.08– 40268 | 20.9– 15320 | 57.1– 31366 |
|
| Czechia | |
Boyin 5 | 21.4–428 |
|
|
|
|
| China | |
Carmagnola | 1.17–1.7 | 4.89–9.1 |
| 13–66.5 |
|
| France | |
|
|
| 6.6–9.9 | 20.2–33.7 | 1.7– 16.5 |
| Italy | |
Carmagnola & Ferimon | 0.14–2.56 | 0.21– 1.88 |
|
|
|
| Italy | |
Chameleon | 3.4 | 7.3 | 83 | 327 | 23 |
| Germany | |
Citrus Sap |
|
| 7.9–11.7 | 20–35 | 2.3– 18.2 |
| Italy | |
Codimono |
| 3.6–12.3 |
| 9.1–62.7 |
|
| Italy | |
Dacia Secuieni | 0.69–1.21 | 2.93–7.5 |
| 7.2–46.5 |
|
| France | |
Denise | 3.1 | 0.24 | 11.8 | 57–59 | 2.1 | 0.88 | Romania | (Mihoc et al., 2012; Mihoc, Pop, Alexa, Dem, & Militaru, 2013) |
Diana | 3 | 0.22 | 10.9 | 52–64 | 6.1 | 0.8 | Romania | |
Fedora 17 | 0.03–2.82 | 0.29– 6.66 | 0.17– 39.2 | 12.4–349 | 0.98– 43.8 | 1.16– 79.1 | Croatia | |
Felina 32 | 3.32 | 4.13 |
| 223.4 |
|
| Italy | |
| 0.16–0.38 | 0.19–0.3 |
|
| 0.59– 1.5 |
| USA | |
Felina 34 |
|
| 7.4– 671.4 | 14.5–28.3 | 3.2–16 |
| Italy | (Angelini et al., 2014; Bona, Marsano, Cavaletto, & Berta, 2007) |
Fibranova | 9.4– 1368.2 |
| 4.78– 11.4 | 14.2–30.2 | 2.7– 321.8 | 1.2–9.0 | Italy Greece | |
Fibranova & Carmagnola |
|
|
|
|
| 0.27– 46.4 | Italy | |
Fibrol | 0.03– 32293 | 0.49– 66280 | 0.17– 29914 | 12.7– 45449 | 1.3– 99.4 | 1.19–272 | Croatia Czechia | |
Futura |
|
| 4–62.5 |
| 14.3– 233 | 0.10– 4.57 | Italy | |
Futura 75 | 0.04–1.11 | 0.54– 13.04 | 0.17– 34.4 | 2.2–689 | 0.13– 55.4 | 1.83– 130.3 | Italy France | |
|
|
|
|
|
|
| Croatia | |
|
|
|
|
|
|
| Greece | |
Futura 75 & Fedora 17 |
|
|
|
|
| 0.74– 100.5 | Italy | |
Henola | 0.31–4.63 |
|
|
|
|
| Poland | |
Huocheng | 17.2–221 |
|
|
|
|
| China | |
Kompolti | 1.4–5.2 |
|
| 410–2600 |
|
| Germany | |
Longxi | 22.8–249 |
|
|
|
|
| China | |
Lu'an | 27.5–218 |
|
|
|
|
| China | |
Monoica | 46.4– 22994 | 6.26– 59837 | 27.9– 27243 | 137– 22564 |
|
| Czechia | |
n/a | 20–1101 |
|
|
|
|
| Pakistan | |
| 2.4–4.4 | 0.42– 1.58 |
|
| 6.8– 10.4 | 15.2– 17.4 | Nigeria | |
| 0.2–1.4 | 0.49– 27.4 | 1.4–24 | 18–220 | 0.1–22 | 0.5–5.0 | Italy | |
| 0.56 | 6.32 | 51.1 | 189.2 | 1.36 |
| Iran | |
|
|
|
|
| 0.09– 2.69 | 0.6–5.4 | Lithuania | |
| 3.2–4.7 | 7.9–10.2 | 122–176 | 315–380 | 124– 172 | 3.6–7.6 | Ethiopia | |
Natural | 0.024–151 | 0.121–46 | 0.002– 1530 | 0.002– 273 | 1.15– 123 | 0.006– 187 | Pakistan | (Mahmood, Rashid, & Malik, 2013; Sulaibi, Thiemann, Thiemann, & T, 2020) |
Qujing | 13.2–310 |
|
|
|
|
| China | |
Red Petiole |
|
| 6.6–9 | 19.1–40 | 1.8– 20.5 |
| Italy | |
Santhica 27 | 0.03–0.36 | 0.57– 14.6 | 0.17– 18.1 | 18.2–69.8 | 0.54– 55.5 | 1.41– 79.4 | Croatia | |
Santhica 70 | 1.1–1.27 | 4.56– 18.26 |
| 12.9– 82.46 |
|
| France | |
Shenmu | 20.3–367 |
|
|
|
|
| China | |
Shenyang | 24.1–360 |
|
|
|
|
| China | |
Shuyang | 14.5–284 |
|
|
|
|
| China | |
Silistrinski | 0.15–1.22 | 1.0–44.8 | 1.3–10.2 | 1.3–78.6 |
|
| Bulgaria | |
Silvana | 3.4 | 0.34 | 12 | 42–69 | 1.6 | 0.82 | Romania | |
Tisza |
|
| 6–480 | 26.5–107 |
|
| Italy | |
USO 14 | 19–357 |
|
|
|
|
| China | |
USO 31 | 11.42 | 10.4 |
| 767 |
|
| Italy | |
| 0.48–0.58 | 11.4– 17.93 |
| 11.97– 46.55 |
|
| France | |
| 0.8–42 | 1.8–22.4 |
|
| 6.9– 63.6 |
| Germany | |
| 333.481 |
|
|
|
|
| China | |
Wuchang 40 | 12.3–217 |
|
|
|
|
| China | |
Xingtai | 12.7–350 |
|
|
|
|
| China | |
Yangcheng | 12.4–398 |
|
|
|
|
| China | |
YM12 | 56.7– 4052.8 |
|
| 80.8– 5030 |
|
| China | |
Yunma 1 | 11.4–340 | 2.97– 275.2 |
|
|
|
| China | |
Yunma 2 | 14.2–268 |
|
|
|
|
| China | |
Yunma 3 | 11.5–350 |
|
|
|
|
| China | |
Yunma 4 | 13.4–368 |
|
|
|
|
| China | |
Zenit | 1.9 | 0.626 | 10.3 | 51–52 | 6 | 0.6 | Romania | |
Zhouqu | 24.9–217 |
|
|
|
|
| China |
The highest Cu concentrations were found in the flower, followed by seeds, roots, stems, leaves, and fibres (Ahmad et al., 2016; Linger, Müssig, Fischer, & Kobert, 2002; Placido & Lee, 2022).
Selenium Phytoremediation
According to research, hemp has the ability to thrive in soil contaminated with selenium (Se) and accumulate selenomethionine and methyl selenocysteine in its seed embryos, indicating that hemp seeds could serve as a source of Se for both humans and livestock (Shi et al., 2012). Additionally, Se can also be found in other above-ground parts of the plant without affecting the quality or yield of extracted metabolites or produced fibres. Furthermore, Se present in the hemp leaves can be utilized as a crop fertilizer for growing in Se-deficient soil (Crini et al., 2021; Malik et al., 2010; Vandenhove & Hees, 2005).
Cadmium, Nickel, and Lead Phytoremediation
Cadmium, nickel, and lead are toxic heavy metals that can accumulate in the environment and pose a threat to human health (Singh et al., 2011). A phytoremediation is a promising approach for the remediation of soil and water contaminated with these heavy metals. Cadmium can be removed from contaminated soil through phytoextraction, where plants such as Indian mustard, sunflowers, and tobacco accumulate high concentrations of cadmium in their shoots, which can be harvested and disposed of (Caldelas, Bort, & Febrero, 2012; Mihoc et al., 2013; Shanker, Cervantes, Loza-Tavera, & Avudainayagam, 2005; Ullah et al., 2019). Nickel phytoremediation can be achieved through a similar mechanism, where plants such as Alyssum accumulate high concentrations of nickel in their shoots. However, nickel phytoremediation is limited by the slow growth rate of the plants and the low nickel concentrations that can be remediated (Arru, Rognoni, Baroncini, Bonatti, & Perata, 2004; Bona et al., 2007).
Lead phytoremediation can be achieved through a combination of phytoextraction, phytostabilization, and rhizofiltration. Plants such as Indian mustard, sunflowers, and vetiver grass can accumulate lead in their shoots, but the effectiveness of phytoextraction is limited by the low solubility of lead in soil (Elisa, Marsano, Cavaletto, & Berta, 2007). Phytostabilization involves the use of plants to reduce the bioavailability of lead by immobilizing it in the soil. Plants such as poplar trees and willows can be used for phytostabilization. Rhizofiltration involves the use of plant roots to filter lead from contaminated water. Plants such as water hyacinth and water lettuce can be used for rhizofiltration (Bañuelos, Terry, Leduc, Pilon-Smits, & Mackey, 2005; Gao et al., 2020; Zhang et al., 2005).
Discussion
The study's findings demonstrate industrial hemp's enormous potential for phytoremediation, particularly when it comes to the buildup of heavy metals in polluted soils. Table 2 shows the significant metal concentrations in different hemp tissues. These results highlight the plant's ability to absorb and sequester toxins, making it an attractive option for environmental remediation. Hemp's deep root system and significant biomass production are important components that guarantee heavy metal removal effectively, which is essential to the success of phytoremediation programmes. The metal amounts that were detected are consistent with well-established phytoremediation processes, providing evidence about the effectiveness of hemp in mitigating environmental contamination. For example, the high zinc (Zn) contents in hemp tissues are consistent with the phytoextraction process, which involves plants absorbing and storing metals in their tissues. Additionally, as mentioned in the literature, the varied amounts of copper (Cu) in various hemp tissues point to a complex interaction between the plant's root system and antioxidant enzymes. The strong biomass production of hemp is correlated with its capacity to remove significant levels of heavy metals, especially lead (Pb) and cadmium (Cd), which highlights the plant's potential as a useful and long-lasting remediation tool. This relationship highlights how crucial plant vigour is to raising the effectiveness of phytoremediation procedures.
An interesting aspect of the research is the examination of possible industrial uses, such as the harvesting of tainted hemp harvests for the creation of biofuel. In addition to being good for the environment, this strategy supports larger goals of lowering dependency on non-renewable energy sources. The feasibility from an economic and environmental standpoint of integrating hemp into biofuel production processes might be the subject of future study in this field. Important problems for real-world applications are raised by the reported variation in metal contents across hemp types. Selecting the best cultivars for a given remediation scenario requires an understanding of how these variables affect hemp growth and output under metal stress. Furthermore, it highlights the necessity of customising phytoremediation techniques to the particular environmental setting in light of the influence of strain characteristics, geographic location, and soil composition on metal concentrations.
Conclusions
In conclusion, cannabis shows promise as a potential phytoremediation agent for heavy metal-contaminated soil. Its ability to absorb and accumulate heavy metals without any significant negative effects on plant growth and yield makes it an attractive option for use in environmental clean-up. Hemp plants possess various mechanisms, such as phytochelatins, antioxidant defence, and compartmentalization, that help in the detoxification and removal of heavy metals from the soil. Furthermore, hemp is a fast-growing crop with high biomass production, which can aid in the efficient removal of heavy metals. While further research is needed to fully explore its potential, it could have significant environmental and industrial applications. However, it is important to consider the safe disposal of contaminated plant material and to ensure that any potential industrial use is done responsibly and sustainably.
Author contributions
Conceptualization, M.S.S., R.K. and K.K.A. Methodology, A.S., A.G.; Validation, T.S., V.S.; Formal analysis A.R.R.; Investigation, A.S., M.S.S., and T.S.; Resources P.K., R.K.; Writing—original draft preparation A.S., V.S. Writing—review and editing, visualization. All authors have read and agreed to the published version of the manuscript.