Introduction
Clostridium botulinum (CB) is a Gram-positive anaerobic bacterium widely distributed in nature, and spores are found in silt sediments, dust and animal faeces. Especially, spores in water and soil are the primary source of food contamination (Tiwari, Nagalli, Clostridium, & Botulinum, 2021). The pathogenicity of CB lies in the neurotoxin botulinum toxin (BTX), a potent neurotoxin known to date (Dong, Masuyer, & Stenmark, 2018). Clinically, botulism caused by BTX is a severe disease that can lead to respiratory distress and death, and cases of foodborne botulism are pretty frequent. The anaerobic growth and low-temperature growth of BTX for toxicity production give it a growth advantage in the increasingly popular vacuum-packed, frozen and canned foods, thus making it one of the significant causes of poisoning from such foods. A study evaluated foodborne botulism from 1955 to 2018 in Ukraine using the National Epidemiological Surveillance Data System. The metadata analyses revealed that during this period (1955-2018), Ukraine recorded 8,614 cases of botulism, resulting in 659 deaths, with self-canned meat and fish being the main contributors to botulism (Semenko et al., 2021). BTX is generally classified into eight (A, B, Cα, Cβ, D, E, F, G) subtypes according to their serotypes and antigenicity. Botulinum toxin type A (BTX-A) is widely used for its high affinity and good tolerability in clinical practice (Satriyasa, 2019). Studies have shown that BTX-A is sustainable and non-addictive in analgesia (Ostrowski, Roszak, & Komisarek, 2009) and has good efficacy in myofascial tension pain, neurogenic pain and inflammatory pain. Because of the discussion above, the current article was designed to review the latest research progress of BTX-A in pain treatment in the last two years.
Myofascial pain
Myofascial pain, affecting both muscles and fascia, is a painful neuromuscular disorder featuring limited muscle pressure. A randomized clinical trial assessed the therapeutic efficacy and safety of BTX-A in treating temporomandibular disorders resulting in persistent myofascial pain. It was found that three different doses of BTX-A (40U, 70U and 100U) had noticeable analgesic effects, but a side effect of reduced electromyographic activity was observed after one month of BTX-A injection, and only the low dose group of BTX-A recovered after three months and returned to baseline levels after six months. Therefore, when considering BTX-A injections for persistent myofascial pain, lower doses of BTX-A are the appropriate choice (La et al., 2020). BTX-A injections for chronic plantar fasciitis were evaluated in a prospective randomised controlled experiment. The BTX-A and placebo groups of 32 patients with persistent plantar fasciitis were randomly assigned, and 70 U of BTX-A were delivered under ultrasound guidance into the medial head of the gastrocnemius muscle. The outcome of the present study showed that local injection of BTX-A into the gastrocnemius muscle had a positive effect on improving pain and foot function in the patients, and the effect lasted for over a year (Abbasian et al., 2019).
The efficacy of BTX-A injections in treating masticatory myofascial pain syndromes (MMPs) is not apparent yet. It is found that minimally invasive strategies and BTX-A injections of the temporalis and occlusal muscles applied to temporomandibular disorder treatment have shown positive results on quality of life and masticatory myofascial pain (Miotto et al., 2021). In a six-month trial, 60 patients with MMPs were randomly assigned to receive saline, lidocaine, or BTX-A, and the effects were assessed on days 7, 14, 28, 60, 90, and 180. The BTX-A group demonstrated significantly less discomfort and improved jaw movement when compared to the saline and lidocaine groups. Additionally, no notable adverse events were seen, implying that BTX-A may be an effective therapy option for individuals with MMPs that are localised (Montes-Carmona, Gonzalez-Perez, & Infante-Cossio, 2020). While the results of this study are promising, further research is needed to determine the long-term advantages of botulinum toxin injections to the masticatory muscles. Platelet-rich plasma and local anaesthetic mepivacaine (LA) were more effective at three months than BTX-A and LA, but only the BTX-A group remained effective at six months. All groups successfully improved symptoms of myofascial trigger points in the masseter muscle with BTX-A and LA being significantly more effective than platelet-rich plasma at three months (Yilmaz, Sivrikaya, Taskesen, Pirpir, & Ciftci, 2020).
A pioneering retrospective study demonstrated that long-term treatment of myofascial neck pain with BTX-A injections is effective and safe, with patients included in the study receiving BTX-A injections at least once a year for a mean duration of 8.3 ± 4.7 years (Diep, Ko, Lan, Koprowicz, & Ko, 2020). The efficacy of BTX-A combined with 0.2% local anaesthetic ropivacaine hydrochloride injection (BTX group) with ropivacaine hydrochloride injection alone (LA group) for the treatment of pelvic floor myofascial syndrome and chronic pelvic pain showing that neither group exhibited any significant differences on day 60. However, the two groups showed an overall improvement in pain, meaning that intramuscular injection with ropivacaine hydrochloride alone was justified (Gorimanipalli, Elavarasi, & Goyal, 2019; Levesque et al., 2021). The randomized clinical trials of BTX-A for myofascial pain has also been elaborated (Table 1).
Table 1
A randomized clinical trial of BTX-A in treating myofascial pain
Inflammatory pain
Knee osteoarthritis
The primary goals of treatment for knee osteoarthritis (KOA) are to alleviate pain, minimise inflammatory response, restore function, and limit disease progression. Currently, the evidence is inconsistent on the efficacy of intra-articular injections of BTX-A in treating KOA. Thirty eligible older persons with KOA received a single injection of 100U of BTX-A solution in a clinical trial. Before and after therapy, the analgesic impact of BTX-A was assessed using the 100 mm visual analogue scale (VAS) and the knee injury and osteoarthritis outcome score (KOOS), respectively. The results showed that BTX-A reduced the subjective pain of KOA patients (Najafi et al., 2019). Another study compared the efficacy of physical therapy, hyaluronic acid intra-articular injections, intra-articular dextrose prolotherapy and BTX-A intra-articular injections for treating KOA in middle-aged and older adults. The outcomes of this study showed better performance in knee pain control in the BTX-A and glucose groups and better efficacy of BTX-A in symptom relief (Rezasoltani et al., 2020). In a meta-analysis comparing the efficacy and safety of intra-articular injections of BTX-A (100U/200U) and placebo in the short-term (≤4 weeks) and long-term (≥8 weeks) treatment of KOA in men, it was also shown that BTX-A is effective and safe in the treatment of KOA (Zhai, Huang, & Yu, 2019), and the above results suggest that BTX-A may have the ability to treat KOA. However, a trial has the opposite conclusion. The researchers found no benefit with BTX-A intra-articular injections for primary KOA versus the use of corticosteroids (tretinoin) and saline. No benefit in improving range of motion, function, pain and quality of life with BTX-A in either the short term (4 weeks) or the medium term (12 weeks) (Mendes et al., 2019). It is unclear whether the dose and duration of treatment affect the efficacy or not. Some reports mention that placebo treatment such as intra-articular injection with saline is also efficacious. However, the articles also point out that the efficacy with saline may lead to opposite conclusions because of differences in study methods (Blanshan & Krug, 2020). Therefore, more high-quality trials and larger sample sizes are needed to confirm the efficacy of intra-articular injections of BTX-A for knee pain.
Lateral epicondylitis
Lateral epicondylitis is a common form of extensor tendinopathy that manifests as discomfort at the origin of the common extensor tendon and pain with resisted wrist extension. BTX-A has been proposed as a therapy option for recalcitrant lateral epicondylitis that has not responded to conservative measures. A recent study injected BTX-A into the common extensor tendon in 60 patients with persistent lateral epicondylitis. The results indicated that both groups saw a considerable drop in numeric rating scale (NRS) values following therapy, with no significant difference in pain alleviation success. However, the high-dose group experienced a greater reduction in NRS scores and a substantial gain in grip strength, indicating that BTX-A injection to the common extensor tendon is a viable therapeutic option for chronic lateral epicondylitis and that high-dose BTX-A is more efficacious (Lee, Choi, & Chang, 2020). This study showed the short-term effects of BTX-A in lateral epicondylitis, but the long-term benefits are unknown. One study evaluated the one-year term efficacy, adverse effects and recurrence rate of BTX-A injections for chronic lateral epicondylitis. Fifty patients were followed up on day 0 (G0), 90 days (G1), 180-270 days (G2), and 365 days (G3) after 40U of BTX-A injected with 40 U of BTX-A. The results showed that patients in the G1, G2, and G3 groups had a significantly better quality of life, reduced pain, and maximum grip strength than the G0 group, with significantly less impact on daily and business activities. Therefore, 1 to 2 injections of 40 U BTX-A were more effective in treating chronic lateral epicondylitis (Cogné et al., 2019). In addition, other studies have also analyzed the effect of different site injections of BTX-A on the efficacy of treatment of lateral epicondylitis, suggesting the location of injection as a potential source of outcome heterogeneity (Song, Day, & Jayaram, 2020).
Shoulder pain
A meta-analysis showed similar outcomes of BTX-A injection compared to the corticosteroid group or saline placebo group in relieving chronic shoulder pain at one month, but its efficacy was better than the other two groups at 1 to 3 months (Hsu, Wu, Han, & Chang, 2020). Another meta-analysis found that shoulder BTX-A injections provided superior analgesia and increased shoulder abduction and external rotation mobility in patients with hemiplegic shoulder pain compared to steroid or placebo injections (Xie et al., 2021). A study comparing the efficacy of pectoralis major and teres major muscle BTX-A injections (BTX-A group) versus suprascapular nerve blocks (SNB group) in treating hemiplegic shoulder pain. The results indicated that the BTX-A group was equally beneficial as the SNB group in alleviating pain, range of motion, and function after two weeks, but was more successful at six weeks (Kasapoğlu-Aksoy, Aykurt-Karlıbel, & Altan, 2020). A 38-year-old man with intractable acromioclavicular joint pain due to distal clavicle osteolysis received an intra-articular injection of BTX-A, experienced a significant reduction in pain, and did not experience pain by avoiding excessive shoulder activity during daily activities (Lee & Park, 2020). The above results indicate that BTX-A injection is a safe and effective alternative therapy for treating patients with shoulder pain. A list of the randomized clinical trials of BTX-A for inflammatory pain has also been tabulated (Table 2).
Table 2
A randomized clinical trial of BTX-A for treating inflammatory pain
Neuropathic pain
Spinal cord injury pain
A trial investigated the effect of subcutaneous injections of BTX-A for treating at-level spinal cord injury pain. Although the trial had a tiny sample size, patients were observed to have varying degrees of improvement in pain, activity, mood and sleep (Chun et al., 2019). A descriptive retrospective study article showed improvement in spasticity (pain, tone and articular limitations) in patients with spinal cord injury given with BTX-A, and no adverse effects linked with post-BTX-A injection were recorded (Palazón-García, Alcobendas-Maestro, Ruz, & AM, 2018). There are hardly any studies that comprehensively analyze the efficacy and safety of BTX-A in treating neuropathic pain after spinal cord injury.
Postherpetic neuralgia
IPost-herpetic neuralgia was studied using the VAS, the effective rate, the McGill pain scale, and the rate of adverse events in a meta-analysis comparing local administration of BTXA with lidocaine. The results showed that patients treated with BTX-A for postherpetic neuralgia had significantly lower VAS pain scores and lower McGill pain questionnaire than those treated with lidocaine at the first month, second month and third-month follow-ups. Furthermore, the effective rate of BTX-A treatment was significantly higher than that of patients receiving lidocaine, with no significant difference in the adverse events rate (Li et al., 2009). Another study also demonstrated that injectable BTX-A was more effective in treating postherpetic neuralgia than oral analgesic gabapentin capsules (Hu et al., 2019), thus indicating that BTX-A may be a very promising drug for the clinical treatment of postherpetic neuralgia.
Diabetic neuropathy pain
In a clinical research, 32 individuals with type 2 diabetes were randomly assigned to receive 100U BTX-A or an equivalent quantity of sodium chloride in the foot. The findings indicated that BTX-A alleviated neuropathic pain and enhanced patients' quality of life and sleep in diabetic neuropathy (Salehi, Moussaei, Kamiab, & Vakilian, 2019). Another prospective experiment evaluated the efficacy of BTX-A in the treatment of diabetic polyneuropathy, in which 141 patients aged 40 to 70 years were randomly assigned to one of three groups: the first group was injected with 150U BTX-A on one side of the foot and 0.9% saline on the other side; the second group was injected with 150U BTX-A in both feet; the third group was injected with 0.9% physiological saline, using VAS and NPS for comparison. The findings indicated a considerable increase in VAS and NPS (except dull and cold sensation) scores in the BTX-A group compared with the saline group, indicating that intradermal injection of BTX-A was effective in treating good diabetic polyneuropathy (Taheri et al., 2020). All of the above results demonstrate that BTX-A effectively improves neuropathic pain in diabetic patients, although different results were shown on NPS, which may be related to sample size and BTX-A dose.
Trigeminal neuralgia
Current studies have shown that BTX-A is an effective and safe treatment for trigeminal neuralgia (Ostrowski et al., 2009; Rubis & Juodzbalys, 2020). A recent study examining factors influencing the efficacy on BTX-A found that a higher success rate of BTX-A treatment was observed in patients aged 50 years or older, but the age factor did not make a significant difference in the time to recurrence of pain, the onset of effect, or peak-time after BTX-A treatment (Wu et al., 2019). Another study indicated that while various doses of BTX-A did not affect the short-term treatment effect, higher levels of BTX-A helped to control trigeminal nerve-induced pain more consistently over time. In addition, the study found that female patients under 70 years of age were more sensitive to BTX-A treatment and concluded that the duration of disease mainly affected the rate of adverse reactions in female patients. However, there was no significant correlation between the duration of disease and the change in side effects in male patients. Therefore, female patients with trigeminal neuralgia with a disease duration of 1-10 months should be more conservative in applying BTX-A, while higher doses of BTX-A injections would have a higher success rate in treating trigeminal neuralgia in male patients (Zhang et al., 2009). A study investigated the safety and efficacy of repeated botulinum toxin injections in the treatment of trigeminal neuralgia. The outcomes of this study revealed that after single and continuous injections of BTX-A, the severity of pain in patients was significantly reduced, the efficiency of pain relief was 100% in both cases, and there was no significant difference in the mean duration of pain relief. No significant adverse effects were seen in either case, suggesting that repeated injections of BTX-A are safe and effective (Gorimanipalli et al., 2019). In addition, some cases have reported that BTX-A is equally effective in trigeminal neuralgia caused by multiple sclerosis, suggesting that BTX-A can also treat atypical trigeminal neuralgia (Calejo, Salgado, Moreira, Correia, & Barros, 2019). Cases of intraoral botulinum toxin injections for trigeminal neuralgia have also been reported, with relief and no facial asymmetry and no adverse effects with repeated intraoral botulinum toxin injections (Dinan, Smith, & Hawkins, 2020; Ostrowski et al., 2009). The list of the randomized clinical trials of BTX-A for neuropathic pain is detailed below (Table 3).
Table 3
A randomized clinical trial of BTX-A in treating neuropathic pain
Conclusion and Prospects
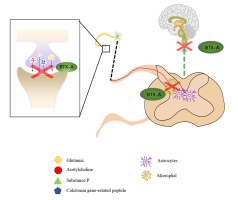
In conclusion, BTX-A, as a new analgesic drug, has definite efficacy in clinical pain management, especially for musculoskeletal, muscle and neuropathic pain. Its advantages include ease of administration, sustainability of efficacy, and no toxic side effects or addiction even after multiple doses within a safe range. However, the application of BTX-A in pain treatment is still in the preliminary stage, and most of the treatments are mainly experience-based. Furthermore, the reports on BTX-A for pain treatment at home and abroad have problems such as a small number of cases and lack of observation of long-term effects, and further research is needed on injection sites and operation techniques. In addition, the effectiveness and side effects of the clinical application of BTX-A in the treatment of pain also need to be confirmed by clinical trials. The solution to these problems requires the accumulation of clinical experience and an accurate grasp of the mechanism of action. Current studies on the analgesic mechanism of BTX-A have focused on two directions: the peripheral nervous system and the central nervous system (Figure 1).
Most studies suggest that BTX-A exerts its analgesic effects by releasing certain transmitters in the peripheral nervous system (such as calcitonin gene-related peptides, substance P and glutamate), anti-inflammatory and other modulation the peripheral nervous system (Lee et al., 2018; Muñoz-Lora et al., 2020; She, Chen, Tang, Chen, & Zheng, 2020). On the other hand, BTX-A exerts its therapeutic effect on pain by activating glial cells such as microglia and astrocytes and regulating the central nervous system-related pathways such as the upstream pain transmission pathway (Shi et al., 2019; Valois, Wilkinson, Nakamura, Henley, & M, 2020). However, to date, the specific targets of BTX-A action are not known, and the most likely targets are specific unknown receptors on the cell. When these targets are activated by binding to BTX-A through a specific pathway, they can produce a sustained effect over a while. BTX-A is well tolerated, with essentially no side effects reported after botulinum toxin injections in the studies included here or only minor adverse effects, such as the appearance of local erythema and facial asymmetry exhibited by injections in the face, and these minor adverse effects disappear within a short period. However, in different clinical trials and in vivo studies, BTX-A injections produced more serious adverse effects, such as reduced masticatory performance and muscle thickness in the occlusal muscles. These adverse events may hinder the benefits of this botulinum toxin. All these questions deserve further exploration in the future.
Conflicts of interest
The authors state that they have no conflict of interest in submitting or publishing this research.
Author contributions
Research concept and design: Yi Cai, Tingdong Yan; Collection and/or assembly of data: Shisheng Jiang, Chaoming Huang, Yantianyu Yang; Data analysis and interpretation: Yantianyu Yang, Shuhan Gao, Zihan Lin, Wei Gu; Writing the article: Shisheng Jiang; Critical revision of the article: Yi Cai, Tingdong Yan; Final approval of the article: Yi Cai, Tingdong Yan